Abstract
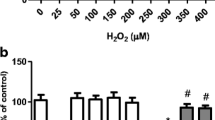
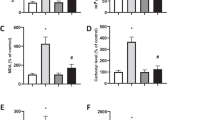
This study was designed to investigate the protective effects of extracellular superoxide dismutase (SOD3) against hydrogen peroxide (H2O2) induced damage in human neuroblastoma SH-SY5Y cells and to elucidate the mechanisms responsible for this beneficial effect. SOD3-overexpressing SH-SY5Y cells were generated by adenoviral vector-mediated infection, and H2O2 was then added into the cell culture system to establish an in vitro model of oxidative stress. Cell viability, the generation of intracellular reactive oxygen species (ROS), the expression and activity of antioxidant enzymes, the levels of lipid peroxidation malondialdehyde (MDA), the expression of mitochondrial apoptosis-related genes, and calcium imaging were examined. Following H2O2 exposure, the over-expression of SOD3 promoted the survival of SH-SY5Y cells; decreased the production of ROS, MDA levels, cytochrome C, caspase-3, caspase-9, and Bax gene expression, and calcium levels; and increased the expression and activity of antioxidant enzyme genes and the expression level of Bcl-2. Together, our data demonstrate that SOD3 ameliorates H2O2-induced oxidative damage in neuroblastoma SH-SY5Y cells by inhibiting the mitochondrial pathway and provide new insights into the functional actions of SOD3 on oxidative stress-induced cell damage.





Similar content being viewed by others
References
Henchcliffe C, Beal MF (2008) Mitochondrial biology and oxidative stress in Parkinson disease pathogenesis. Nat Clin Pract 4:600–609. doi:10.1038/ncpneuro0924
Behl C (1999) Vitamin E and other antioxidants in neuroprotection. Int J Vitam Nutr Res 69:213–219. doi:10.1024/0300-9831.69.3.213
Finkel T, Holbrool NJ (2000) Oxidants, oxidative stress and the biology of ageing. Nature 408:239–247. doi:10.1038/35041687
Lobo V, Patil A, Phatak A, Chandra N (2010) Free radicals, antioxidants and functional foods: impact on human health. Pharmacogn Rev 4:118–126. doi:10.4103/0973-7847.70902
Manczak M, Park BS, Jung Y, Reddy PH (2004) Differential expression of oxidative phosphorylation genes in patients with Alzheimer’s disease: implications for early mitochondrial dysfunction and oxidative damage. NeuroMol Med 5:147–162. doi:10.1385/NMM:5:2:147
Fukai T, Folz RJ, Landmesser U, Harrison DG (2002) Extracellular superoxide dismutase and cardiovascular disease. Cardiovasc Res 55:239–249. doi:10.1016/S0008-6363(02)00328-0
Zelko IN, Mariani TJ, Folz RJ (2002) Superoxide dismutase multigene family: a comparison of the CuZn-SOD (SOD1), Mn-SOD (SOD2), and EC-SOD (SOD3) gene structures, evolution, and expression. Free Radic Biol Med 33:337–349. doi:10.1016/S0891-5849(02)00905-X
Miao L, St Clair DK (2009) Regulation of superoxide dismutase genes: implications in disease. Free Radic Biol Med 47:344–356. doi:10.1016/j.freeradbiomed.2009.05.018
Chelikani P, Fita I, Loewen PC (2004) Diversity of structures and properties among catalases. Cell Mol Life Sci CMLS 61:192–208. doi:10.1007/s00018-003-3206-5
Miguel F, Augusto AC, Gurgueira SA (2009) Effect of acute vs chronic H2O2-induced oxidative stress on antioxidant enzyme activities. Free Radical Res 43:340–347. doi:10.1080/10715760902751894
Fukui M, Zhu BT (2010) Mitochondrial superoxide dismutase SOD2, but not cytosolic SOD1, plays a critical role in protection against glutamate-induced oxidative stress and cell death in HT22 neuronal cells. Free Radic Biol Med 48:821–830. doi:10.1016/j.freeradbiomed.2009.12.024
Marklund SL (1984) Extracellular superoxide dismutase in human tissues and human cell lines. J Clin Investig 74:1398–1403. doi:10.1172/JCI111550
Van Rheen Z, Fattman C, Domarski S, Majka S, Klemm D, Stenmark KR, Nozik-Grayck E (2011) Lung extracellular superoxide dismutase overexpression lessens bleomycin-induced pulmonary hypertension and vascular remodeling. Am J Respir Cell Mol Biol 44:500–508. doi:10.1165/rcmb.2010-0065OC
Slater AF, Stefan C, Nobel I, van den Dobbelsteen DJ, Orrenius S (1996) Intracellular redox changes during apoptosis. Cell Death Differ 3:57–62
Anderson KM, Seed T, Ou D, Harris JE (1999) Free radicals and reactive oxygen species in programmed cell death. Med Hypotheses 52:451–463. doi:10.1054/mehy.1997.0521
Curtin JF, Donovan M, Cotter TG (2002) Regulation and measurement of oxidative stress in apoptosis. J Immunol Methods 265:49–72. doi:10.1016/S0022-1759(02)00070-4
Sinha K, Das J, Pal PB, Sil PC (2013) Oxidative stress: the mitochondria-dependent and mitochondria-independent pathways of apoptosis. Arch Toxicol 87:1157–1180. doi:10.1007/s00204-013-1034-4
Green DR, Kroemer G (2004) The pathophysiology of mitochondrial cell death. Science 305:626–629. doi:10.1126/science.1099320
Ho YS, Crapo JD (1988) Isolation and characterization of complementary DNAs encoding human manganese-containing superoxide dismutase. FEBS Lett 229:256–260. doi:10.1016/0014-5793(88)81136-0
Kasahara E, Lin LR, Ho YS, Reddy VN (2005) SOD2 protects against oxidation-induced apoptosis in mouse retinal pigment epithelium: implications for age-related macular degeneration. Invest Ophthalmol Vis Sci 46:3426–3434. doi:10.1167/iovs.05-0344
Huang TT, Zou Yani, Corniola Rikki (2012) Oxidative stress and adult neurogenesis–effects of radiation and superoxide dismutase deficiency. Semin Cell Dev Biol 23:738–744. doi:10.1016/j.semcdb.2012.04.003
Soriano ME, Scorrano L (2011) Traveling Bax and forth from mitochondria to control apoptosis. Cell 145:15–17. doi:10.1016/j.cell.2011.03.025
Allan SM, Rothwell NJ (2001) Cytokines and acute neurodegeneration. Nat Rev Neurosci 2:734–744. doi:10.1038/35094583
Sei Y, Vitkovic L, Yokoyama MM (1995) Cytokines in the central nervous system: regulatory roles in neuronal function, cell death and repair. NeuroImmunoModulation 2:121–133
Kuhn TB (2014) Oxygen radicals elicit paralysis and collapse of spinal cord neuron growth cones upon exposure to proinflammatory cytokines. BioMed Res Int. doi:10.1155/2014/191767
Liochev SI, Fridovich I (2002) The Haber-Weiss cycle—70 years later: an alternative view. Redox Rep Commun Free Radic Res 7:55–57. doi:10.1179/135100002125000190
von Sonntag C (2008) Advanced oxidation processes: mechanistic aspects. Water Sci Technol 58:1015–1021. doi:10.2166/wst.2008.467
Azmi NH, Ismail N, Imam MU, Ismail M (2013) Ethyl acetate extract of germinated brown rice attenuates hydrogen peroxide-induced oxidative stress in human SH-SY5Y neuroblastoma cells: role of anti-apoptotic, pro-survival and antioxidant genes. BMC Complement Altern Med 13:177–188. doi:10.1186/1472-6882-13-177
Ismail N, Ismail M, Imam MU, Azmi NH, Fathy SF, Foo JB, Abu Bakar MF (2014) Mechanistic basis for protection of differentiated SH-SY5Y cells by oryzanol-rich fraction against hydrogen peroxide-induced neurotoxicity. BMC Complement Altern Med 14:467–478. doi:10.1186/1472-6882-14-467
Stefanova NA, Kozhevnikova OS, Vitovtov AO, Maksimova KY, Logvinov SV, Rudnitskaya EA, Korbolina EE, Muraleva NA, Kolosova NG (2014) Senescence-accelerated OXYS rats: a model of age-related cognitive decline with relevance to abnormalities in Alzheimer disease. Cell Cycle 13:898–909. doi:10.4161/cc.28255
Rusnak F, Mertz P (2000) Calcineurin: form and function. Physiol Rev 80:1483–1521
Holley AK, Dhar SK, St Clair DK (2010) Manganese superoxide dismutase versus p53: the mitochondrial center. Ann N Y Acad Sci 1201:72–78. doi:10.1111/j.1749-6632.2010.05612.x
Przedborski S, Kostic V, Jackson-Lewis V, Naini AB, Simonetti S, Fahn S, Carlson E, Epstein CJ, Cadet JL (1992) Transgenic mice with increased Cu/Zn-superoxide dismutase activity are resistant to N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced neurotoxicity. J Neurosci 12:1658–1667
Mondola P, Annella T, Santillo M et al (1996) Evidence for secretion of cytosolic CuZn superoxide dismutase by Hep G2 cells and human fibroblasts. Int J Biochem Cell Biol 28:677–681. doi:10.1016/1357-2725(96)00004-0
Mondola P, Ruggiero G, Serù R, Damiano S et al (2003) The Cu, Zn superoxide dismutase in neuroblastoma SK-N-BE cells is exported by a microvesicles dependent pathway. Brain Res Mol Brain Res 110:45–51. doi:10.1016/S0169-328X(02)00583-1
Baines CP (2010) Role of the mitochondrion in programmed necrosis. Front Physiol 1:156. doi:10.3389/fphys.2010.00156
Ebenezer PJ, Weidner AM, LeVine H 3rd, Markesbery WR et al (2010) Neuron specific toxicity of oligomeric amyloid-beta: role for JUN-kinase and oxidative stress. J Alzheimer’s Dis JAD 22:839–848. doi:10.3233/JAD-2010-101161
Zhang L, Wei S, Tang JM, Guo LY et al (2013) PEP-1-CAT protects hypoxia/reoxygenation-induced cardiomyocyte apoptosis through multiple sigaling pathways. J Transl Med 11:113. doi:10.1186/1479-5876-11-113
Yan MH, Wang X, Zhu X (2013) Mitochondrial defects and oxidative stress in Alzheimer disease and Parkinson disease. Free Radic Biol Med 62:90–101. doi:10.1016/j.freeradbiomed.2012.11.014
Zhang JQ, Shen M, Zhu CC, Yu FX, Liu ZQ, Ally N, Sun SC, Li K, Liu HL (2014) 3-Nitropropionic acid induces ovarian oxidative stress and impairs follicle in mouse. PLoS ONE 9:e86589. doi:10.1371/journal.pone.0086589
Liochev SI (2013) Reactive oxygen species and the free radical theory of aging. Free Radic Biol Med 60:1–4. doi:10.1016/j.freeradbiomed.2013.02.011
Chaudhari N, Talwar P, Parimisetty A, Lefebvre d’ Hellencourt C, Ravanan P (2014) A molecular web: endoplasmic reticulum stress, inflammation, and oxidative stress. Front Cell Neurosci 8:213. doi:10.3389/fncel.2014.00213
Paulsen Bda S, de Moraes Maciel R, Galina A, Souza da Silveira M, dos Santos Souza C, Drummond H, Nascimento Pozzatto E, Silva H Jr, Chicaybam L, Massuda R, Setti-Perdigão P, Bonamino M, Belmonte-de-Abreu PS, Castro NG, Brentani H, Rehen SK (2012) Altered oxygen metabolism associated to neurogenesis of induced pluripotent stem cells derived from a schizophrenic patient. Cell Transp 21:1547–1559. doi:10.3727/096368911X600957
Mattson MP (2007) Calcium and neurodegeneration. Aging Cell 6:337–350. doi:10.1111/j.1474-9726.2007.00275.x
Khachaturian ZS (1987) Hypothesis on the regulation of cytosol calcium concentration and the aging brain. Neurobiol Aging 8:345–346. doi:10.1016/0197-4580(87)90073-X
LaFerla FM (2002) Calcium dyshomeostasis and intracellular signalling in Alzheimer’s disease. Nat Rev Neurosci 3:862–872. doi:10.1038/nrn960
Acknowledgments
This work was supported mainly by the Chongqing Science and Technology Committee Fund for Cutting-Edge Research Projects (CSTC2014JCYJA10014) and a grant from the Scientific and Technological Research Program of Chongqing Municipal Education Commission (KJ130320). It was also partially supported by grants from the National Natural Science Foundation of China (NSFC Grant Numbers 81001220 and 81370077), and the Fundamental Research Funds for nonprofit public scientific research institutions of Chongqing from Chongqing Science and Technology Commission (Grant no. 2015CSTC-JBKY-01702).
Author Contributions
HY. and H.R.Y. designed this study; R.Y., L.W., Q.Q.F., and H.W. performed the experiments; R.Y. analyzed the data; R.Y. and H.Y. wrote the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing financial interests.
Additional information
Rong Yang and Li Wei have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Yang, R., Wei, L., Fu, QQ. et al. SOD3 Ameliorates H2O2-Induced Oxidative Damage in SH-SY5Y Cells by Inhibiting the Mitochondrial Pathway. Neurochem Res 41, 1818–1830 (2016). https://doi.org/10.1007/s11064-016-1897-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-016-1897-x