Abstract
Mitochondria are a primary source and a target of reactive oxygen species (ROS). Increased mitochondrial production of ROS is associated with bioenergetics decline, cell death, and inflammation. Here we investigated whether a pretreatment (for 24 h) with sesamol (SES; at 12.5—50 µM) would be efficient in preventing the mitochondrial collapse induced by hydrogen peroxide (H2O2, at 300 µM) in the human neuroblastoma SH-SY5Y cell line. We have found that a pretreatment with SES at 25 µM decreased the effects of H2O2 on lipid peroxidation, protein carbonylation, and protein nitration in membranes obtained from the mitochondria isolated from the SH-SY5Y cells. In this regard, SES pretreatment decreased the production of superoxide anion radical (O2−•) by the mitochondria of H2O2-treated cells. SES also prevented the mitochondrial dysfunction induced by H2O2, as assessed by analyzing the activity of the complexes I and V. The H2O2-induced reduction in the production of adenosine triphosphate (ATP) was also prevented by SES. The levels of the pro-inflammatory cytokines interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α), as well as the activity of the transcription factor nuclear factor-κB (NF-κB) were downregulated by the SES pretreatment in the H2O2-challenged cells. Silencing of the nuclear factor erythroid 2-related factor 2 (Nrf2) transcription factor abolished the protection induced by SES regarding mitochondrial function and inflammation. Thus, SES depends on Nrf2 to promote mitochondrial protection in cells facing redox impairment.








Similar content being viewed by others
Data availability
All data generated or analysed during this study are included in this published article (and its supplementary information files).
References
Adam-Vizi V (2005) Production of reactive oxygen species in brain mitochondria: contribution by electron transport chain and non-electron transport chain sources. Antioxid Redox Signal 7(9–10):1140–1149. https://doi.org/10.1089/ars.2005.7.1140
Borutaite V, Morkuniene R, Brown GC (1999) Release of cytochrome c from heart mitochondria is induced by high Ca2+ and peroxynitrite and is responsible for Ca(2+)-induced inhibition of substrate oxidation. Biochim Biophys Acta 1453:41–48. https://doi.org/10.1016/s0925-4439(98)00082-9
Brand MD (2016) Mitochondrial generation of superoxide and hydrogen peroxide as the source of mitochondrial redox signaling. Free Radic Biol Med 100:14–31. https://doi.org/10.1016/j.freeradbiomed.2016.04.001
Coleman JW (2001) Nitric oxide in immunity and inflammation. Int Immunopharmacol 1:1397–1406. https://doi.org/10.1016/s1567-5769(01)00086-8
de Oliveira MR (2018) Carnosic acid as a promising agent in protecting mitochondria of brain cells. Mol Neurobiol 55:6687–6699. https://doi.org/10.1007/s12035-017-0842-6
de Oliveira MR, Nabavi SM, Braidy N, Setzer WN, Ahmed T, Nabavi SF (2016) Quercetin and the mitochondria: a mechanistic view. Biotechnol Adv 34:532–549. https://doi.org/10.1016/j.biotechadv.2015.12.014
de Oliveira MR, da Costa FG, Peres A, Bosco SMD (2018a) Carnosic acid suppresses the H2O2-induced mitochondria-related bioenergetics disturbances and redox impairment in SH-SY5Y cells: role for Nrf2. Mol Neurobiol 55:968–979. https://doi.org/10.1007/s12035-016-0372-7
de Oliveira MR, de Souza ICC, Fürstenau CR (2018b) Carnosic acid induces anti-inflammatory effects in paraquat-treated SH-SY5Y cells through a mechanism involving a crosstalk between the Nrf2/HO-1 axis and NF-κB. Mol Neurobiol 55:890–897. https://doi.org/10.1007/s12035-017-0389-6
de Oliveira MR, Brasil FB, Fürstenau CR (2019) Nrf2 mediates the anti-apoptotic and anti-inflammatory effects induced by gastrodin in hydrogen peroxide-treated SH-SY5Y cells. J Mol Neurosci 69:115–122. https://doi.org/10.1007/s12031-019-01339-3
Duarte AR, Chenet AL, Souza de Almeida FJ, Balbinotti Andrade CM, Roberto de Oliveira M (2018) The inhibition of heme oxigenase-1 (HO-1) abolishes the mitochondrial protection induced by sesamol in LPS-treated RAW 264.7 cells. Chem Biol Interact 296:171–178. https://doi.org/10.1016/j.cbi.2018.09.012
Gupta A, Sharma S, Kaur I, Chopra K (2009) Renoprotective effects of sesamol in ferric nitrilotriacetate-induced oxidative renal injury in rats. Basic Clin Pharmacol Toxicol 104:316–321. https://doi.org/10.1111/j.1742-7843.2009.00381.x
Hayes JD, Flanagan JU, Jowsey IR (2005) Glutathione transferases. Annu Rev Pharmacol Toxicol 45:51–88. https://doi.org/10.1146/annurev.pharmtox.45.120403.095857
Holmström KM, Kostov RV, Dinkova-Kostova AT (2016) The multifaceted role of Nrf2 in mitochondrial function. Curr Opin Toxicol 1:80–91. https://doi.org/10.1016/j.cotox.2016.10.002
Hsu DZ, Chien SP, Chen KT, Liu MY (2007) The effect of sesamol on systemic oxidative stress and hepatic dysfunction in acutely iron-intoxicated mice. Shock 28:596–601. https://doi.org/10.1097/shk.0b013e31804d4474
Joshi R, Kumar MS, Satyamoorthy K, Unnikrisnan MK, Mukherjee T (2005) Free radical reactions and antioxidant activities of sesamol: pulse radiolytic and biochemical studies. J Agric Food Chem 53:2696–2703. https://doi.org/10.1021/jf0489769
Kalyanaraman B (2013) Teaching the basics of redox biology to medical and graduate students: oxidants, antioxidants and disease mechanisms. Redox Biol 1:244–257. https://doi.org/10.1016/j.redox.2013.01.014
Kamsler A, Segal M (2004) Hydrogen peroxide as a diffusible signal molecule in synaptic plasticity. Mol Neurobiol 29:167–178. https://doi.org/10.1385/MN:29:2:167
Kudin AP, Bimpong-Buta NY, Vielhaber S, Elger CE, Kunz WS (2004) Characterization of superoxide-producing sites in isolated brain mitochondria. J Biol Chem 279:4127–4135. https://doi.org/10.1074/jbc.M310341200
Kudin AP, Debska-Vielhaber G, Kunz WS (2005) Characterization of superoxide production sites in isolated rat brain and skeletal muscle mitochondria. Biomed Pharmacother 59:163–168. https://doi.org/10.1016/j.biopha.2005.03.012
Liu T, Zhang L, Joo D, Sun SC (2017) NF-κB signaling in inflammation. Signal Transduct Target Ther 2:17023. https://doi.org/10.1038/sigtrans.2017.23
Mensor LL, Menezes FS, Leitão GG, Reis AS, dos Santos TC, Coube CS, Leitão SG (2001) Screening of Brazilian plant extracts for antioxidant activity by the use of DPPH free radical method. Phytother Res 15:127–130. https://doi.org/10.1002/ptr.687
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63. https://doi.org/10.1016/0022-1759(83)90303-4
Naik E, Dixit VM (2011) Mitochondrial reactive oxygen species drive proinflammatory cytokine production. J Exp Med 208:417–420. https://doi.org/10.1084/jem.20110367
Naoi M, Maruyama W, Shamoto-Nagai M, Yi H, Akao Y, Tanaka M (2005) Oxidative stress in mitochondria: decision to survival and death of neurons in neurodegenerative disorders. Mol Neurobiol 31:81–93. https://doi.org/10.1385/MN:31:1-3:081
Nunes-Nesi A, Araújo WL, Obata T, Fernie AR (2013) Regulation of the mitochondrial tricarboxylic acid cycle. Curr Opin Plant Biol 16:335–343. https://doi.org/10.1016/j.pbi.2013.01.004
Papa S, Martino PL, Capitanio G, Gaballo A, De Rasmo D, Signorile A, Petruzzella V (2012) The oxidative phosphorylation system in mammalian mitochondria. Adv Exp Med Biol 942:3–37. https://doi.org/10.1007/978-94-007-2869-1_1
Pavlin M, Repič M, Vianello R, Mavri J (2016) The chemistry of neurodegeneration: kinetic data and their implications. Mol Neurobiol 53:3400–3415. https://doi.org/10.1007/s12035-015-9284-1
Poderoso JJ, Carreras MC, Lisdero C, Riobó N, Schöpfer F, Boveris A (1996) Nitric oxide inhibits electron transfer and increases superoxide radical production in rat heart mitochondria and submitochondrial particles. Arch Biochem Biophys 28:85–92. https://doi.org/10.1006/abbi.1996.0146
Prasad NR, Mahesh T, Menon VP, Jeevanram RK, Pugalendi KV (2005) Photoprotective effect of sesamol on UVB-radiation induced oxidative stress in human blood lymphocytes in vitro. Environ Toxicol Pharmacol 20:1–5. https://doi.org/10.1016/j.etap.2004.09.009
Radi R (2013) Protein tyrosine nitration: biochemical mechanisms and structural basis of functional effects. Acc Chem Res 46:550–559. https://doi.org/10.1021/ar300234c
Ren B, Yuan T, Diao Z, Zhang C, Liu Z, Liu X (2018) Protective effects of sesamol on systemic oxidative stress-induced cognitive impairments via regulation of Nrf2/Keap1 pathway. Food Funct 9:5912–5924. https://doi.org/10.1039/c8fo01436a
Sies H, Berndt C, Jones DP (2017) Oxidative Stress. Annu Rev Biochem 86:715–748. https://doi.org/10.1146/annurev-biochem-061516-045037
Sinha K, Das J, Pal PB, Sil PC (2013) Oxidative stress: the mitochondria-dependent and mitochondria-independent pathways of apoptosis. Arch Toxicol 87:1157–1180. https://doi.org/10.1007/s00204-013-1034-4
Soufli I, Toumi R, Rafa H, Touil-Boukoffa C (2016) Overview of cytokines and nitric oxide involvement in immuno-pathogenesis of inflammatory bowel diseases. World J Gastrointest Pharmacol Ther 7:353–360. https://doi.org/10.4292/wjgpt.v7.i3.353
VanGilder RL, Huber JD (2014) Sesamol: a treatment for diabetes-associated blood-brain barrier dysfunction. Postdoc J 2:13–22
Waghray M, Cui Z, Horowitz JC, Subramanian IM, Martinez FJ, Toews GB, Thannickal VJ (2005) Hydrogen peroxide is a diffusible paracrine signal for the induction of epithelial cell death by activated myofibroblasts. FASEB J 19:854–586. https://doi.org/10.1096/fj.04-2882fje
Wakabayashi N, Slocum SL, Skoko JJ, Shin S, Kensler TW (2010) When NRF2 talks, who’s listening? Antioxid Redox Signal 13:1649–1663. https://doi.org/10.1089/ars.2010.3216
Wang C, Youle RJ (2009) The role of mitochondria in apoptosis*. Annu Rev Genet 43:95–118. https://doi.org/10.1146/annurev-genet-102108-134850
Wang K, Zhu L, Zhu X, Zhang K, Huang B, Zhang J, Zhang Y, Zhu L, Zhou B, Zhou F (2014) Protective effect of paeoniflorin on Aβ25-35-induced SH-SY5Y cell injury by preventing mitochondrial dysfunction. Cell Mol Neurobiol 34:227–234. https://doi.org/10.1007/s10571-013-0006-9
Wu XL, Liou CJ, Li ZY, Lai XY, Fang LW, Huang WC (2015) Sesamol suppresses the inflammatory response by inhibiting NF-κB/MAPK activation and upregulating AMP kinase signaling in RAW 264.7 macrophages. Inflamm Res 64:577–588. https://doi.org/10.1007/s00011-015-0836-7
Zorova LD, Popkov VA, Plotnikov EY, Silachev DN, Pevzner IB, Jankauskas SS, Babenko VA, Zorov SD, Balakireva AV, Juhaszova M, Sollott SJ, Zorov DB (2018) Mitochondrial membrane potential. Anal Biochem 552:50–59. https://doi.org/10.1016/j.ab.2017.07.009
Acknowledgements
This work was supported by the Conselho Nacional de Pesquisa e Desenvolvimento Tecnológico (CNPq; Edital Universal 2016 protocol number 400216/2016-7). MRO receives a “Bolsista Produtividade PQ2” fellow (protocol number 301273/2018-9). SMSN received a fellow from the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES).
Funding
This study was funded by Conselho Nacional de Pesquisa e Desenvolvimento Tecnológico (CNPq) [grant number 301273/2018-9 (Bolsa de Produtividade em Pesquisa 2-PQ2) and grant number 400216/2016-7].
Author information
Authors and Affiliations
Contributions
Conceptualization: Marcos Roberto de Oliveira; Methodology: Sônia Mendes da Silva Navarro, Fhelipe Jolner Souza de Almeida, Matheus Dargesso Luckachaki, Marcos Roberto de Oliveira; Formal analysis and investigation: Sônia Mendes da Silva Navarro, Fhelipe Jolner Souza de Almeida, Matheus Dargesso Luckachaki, Marcos Roberto de Oliveira; Writing—original draft preparation: Marcos Roberto de Oliveira; Writing—review and editing: Sônia Mendes da Silva Navarro, Fhelipe Jolner Souza de Almeida, Matheus Dargesso Luckachaki, Marcos Roberto de Oliveira; Funding acquisition: Marcos Roberto de Oliveira; Resources: Marcos Roberto de Oliveira; Supervision: Marcos Roberto de Oliveira.
Corresponding author
Ethics declarations
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
11011_2021_875_MOESM1_ESM.pdf
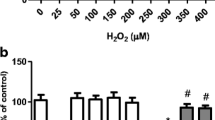
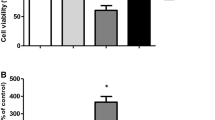
Supplementary file1 Figure S1. Quality control for the extraction of mitochondria from the SH-SY5Y cells. The activity of the lactate dehydrogenase (LDH), which is located in the cytosol of mammalian cells, was checked aiming to evaluate whether the mitochondrial and nuclear samples were contaminated by cytosolic components. Figure S2. Quality control for the silencing of Nrf2. The cells were treated with SES at 25 µM for 24 h and the activity of the transcription factor Nrf2 was accessed by using a commercial assay kit, as described in the Material and Methods section. Data are shown as the mean ± S.D. of five independent experiments each done in triplicate. One-way ANOVA followed by the post hoc Tukey’s test, * p < 0.05 different from control cells transfected with negative control (NC) siRNA; # p < 0.05 different from SES-treated cells transfected with NC siRNA. Figure S3. SES prevented the effects of H2O2 on the viability of the SH-SY5Y cells. SES was tested at 12.5 - 50 μM for 24 h before the exposure to H2O2 at 300 μM for further 24 h. Data are shown as the mean ± S.D. of five independent experiments each done in triplicate. One-way ANOVA followed by the post hoc Tukey's test, * p < 0.05 different from the control group; # p < 0.05 different from H2O2-treated group. Figure S4. The mitochondria-related anti-apoptotic effects induced by SES pretreatment in H2O2-challenged SH-SY5Y cells. The levels of Bcl-2 (A), Bax (B), and cytosolic (C) and mitochondrial cytochrome c (D), as well as the activity of the caspases-9 (E) and -3 (F), were modulated by SES in the cells exposed to H2O2. SES at 25 μM was administrated to the cells for 24 h before the challenge with H2O2 at 300 μM for further 24 h. Data are shown as the mean ± S.D. of five independent experiments each done in triplicate. One-way ANOVA followed by the post hoc Tukey's test, * p < 0.05 different from the control group; # p < 0.05 different from H2O2-treated group. Figure S5. SES decreased the oxidation of DPPH. SES at 25 and 50 µM inhibited the oxidation of DPPH. Data are shown as the mean ± S.D. of five independent experiments each done in triplicate. One-way ANOVA followed by the post hoc Tukey's test, * p < 0.05 different from the control group. Figure S6. Nrf2 silencing abrogated the reduction elicited by SES on the mitochondrial production of O2-• in H2O2-challenged cells. Data are shown as the mean ± S.D. of five independent experiments each done in triplicate. One-way ANOVA followed by the post hoc Tukey’s test, a p < 0.05 different from the control; b p < 0.05 different from H2O2-challenged cells transfected with negative control (NC) siRNA; * p < 0.05 different from H2O2-challenged cells transfected with NC siRNA; # p < 0.05 different from SES + H2O2-treated cells transfected with NC siRNA (PDF 243 KB)
Rights and permissions
About this article
Cite this article
da Silva Navarro, S.M., de Almeida, F.J.S., Luckachaki, M.D. et al. Sesamol prevents mitochondrial impairment and pro-inflammatory alterations in the human neuroblastoma SH-SY5Y cells: role for Nrf2. Metab Brain Dis 37, 607–617 (2022). https://doi.org/10.1007/s11011-021-00875-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-021-00875-5