Abstract
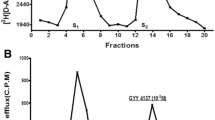
Rat posterior eyecups containing the retina were prepared, loaded with [3H]glycine and superfused in order to determine its release originated from glycinergic amacrine cells and/or glial cells. Deprivation of oxygen and glucose from the Krebs-bicarbonate buffer used for superfusion evoked a marked increase of [3H]glycine release, an effect that was found to be external Ca2+-independent. Whereas oxygen and glucose deprivation increased [3H]glycine release, its uptake was reduced suggesting that energy deficiency shifts glycine transporter type-1 operation from normal to reverse mode. The increased release of [3H]glycine evoked by oxygen and glucose deprivation was suspended by addition of the non-competitive glycine transporter type-1 inhibitor NFPS and the competitive inhibitor ACPPB further suggesting the involvement of this transporter in the mediation of [3H]glycine release. Oxygen and glucose deprivation also evoked [3H]glutamate release from rat retina and the concomitantly occurring release of the NMDA receptor agonist glutamate and the coagonist glycine makes NMDA receptor pathological overstimulation possible in hypoxic conditions. [3H]Glutamate release was suspended by addition of the excitatory amino acid transporter inhibitor TBOA. Sarcosine, a substrate inhibitor of glycine transporter type-1, also increased [3H]glycine release probably by heteroexchange shifting transporter operation into reverse mode. This effect of sarcosine was also external Ca2+-independent and could be suspended by NFPS. Energy deficiency in retina induced by ouabain, an inhibitor of the Na+–K+-dependent ATPase, and by rotenone, a mitochondrial complex I inhibitor added with the glycolytic inhibitor 2-deoxy-d-glucose, led to increase of retinal [3H]glycine efflux. These effects of ouabain and rotenone/2-deoxy-d-glucose could also be blocked by NFPS pointed to the preferential reverse mode operation of glycine transporter type-1 as a consequence of impaired cellular energy homeostasis. Immunohistochemical studies revealed that glycine transporter type-1, of which reverse mode operation assures [3H]glycine release, is expressed in amacrine cells in the inner nuclear and plexiform layers of the retina and also in Müller macroglia cells. We conclude that disruption of the balanced normal/reverse mode operation of glycine transporter type-1 is likely a significant factor contributing to neurotoxic processes of the retina. The possibility to inhibit glycine transporter type-1 mediated glycine efflux by drugs more potently than glycine uptake might offer some therapeutic potential for the treatment of various neurodegenerative disorders of the retina.






Similar content being viewed by others
References
Johnson JW, Ascher P (1987) Glycine potentiates the NMDA response in cultured mouse brain neurons. Nature 325:529–537
Wässle H, Heinze L, Ivanova I, Majumdar S, Weiss J, Harvey RJ, Havenkamp S (2009) Glycinergic transmission in the mammalian retina. Front Mol Neurosci 2:6–20
Pow DV, Hendrickson AE (1999) Distribution of the glycine transporter glyt-1 in mammalian and nonmammalian retinae. Vis Neurosci 16:231–239
Haverskomp S, Wässle H (2000) Immunohistochemical analysis of the mouse retina. J Comp Neurol 424:1–23
Gadea A, Lopez E, Lopez-Colome AM (1999) Characterization of glycine transport in cultured Muller glial cells from the retina. Glia 26:273–279
Lee S-C, Zhong Y-M, Yang X-L (2005) Expression of glycine receptor and transporter on bullfrog retinal Müller cells. Neurosci Lett 387:75–79
Osborne NN, Casson RJ, Wood JPM, Chidlow G, Graham M, Melena J (2004) Retinal ischemia: mechanisms of damage and potential therapeutic strategies. Prog Retinal Eye Res 23:91–147
Beart PM, O’Shea RD (2007) Transporters for L-glutamate: an update on their molecular pharmacology and pathological involvement. Br J Pharmacol 150:5–17
Harsing LG Jr, Albert M, Matyus P, Szenasi G (2012) Inhibition of hypoxia-induced [3H]glycine release from chicken retina by the glycine transporter type-1 (GlyT-1) inhibitors NFPS and Org-24461. Exp Eye Res 94:6–12
Barnett NL, Pow DV, Bull ND (2001) Differential perturbation of neuronal and glial glutamate transport systems in retinal ischemia. Neurochem Int 39:291–299
Phillis JW, Regan MH (2003) Characterization of modes of release of amino acids in the ischemic/reperfused rat cerebral cortex. Neurochem Int 43:461–467
Thomsen C (2006) Glycine transporter inhibitors as novel antipsychotics. Drug Discov Today Ther Strateg 3:539–545
Grewer C, Gameiro A, Zhang Z, Tao Z, Braams S, Rauen T (2009) Glutamate forward and reverse transport: from molecular mechanism to transporter-mediated release after ischemia. IUBMB Life 60:609–619
Fletcher EL, Hack I, Brandstätter JH, Wässle H (2000) Synaptic localization of NMDA receptor subunits in rat retina. J Comp Neurol 420:98–112
Hama Y, Katsuki H, Tochikawa Y, Suminaka C, Kume T, Akaike A (2006) Contribution of endogenous glycine site NMDA agonists to excitotoxic retinal damage in vivo. Neurosci Res 56:279–285
Chidlow G, Wood JPM, Casson RJ (2007) Pharmacological neuroprotection for glaucoma. Drugs 67:725–759
Schmidt K-G, Bergert H, Funk RHW (2008) Neurodegenerative diseases of the retina and potential for protection and recovery. Curr Neuropharmacol 6:164–178
Osborne NN, Melena J, Chidrow G, Wood JPM (2001) A hypothesis to explain ganglion cell death caused by vascular insults at the optic nerve head: possible implications for the treatment of glaucoma. Br J Ophthalmol 85:1252–1259
Smith KE, Borden LA, Hartig PA, Branchek T, Weinshank RL (1992) Cloning and expression of a glycine transporter reveal colocalization with NMDA receptors. Neuron 8:927–936
Cubelos B, Gimenez C, Zafra F (2005) Localization of the GLYT1 glycine transporter at glutamatergic synapses in the rat brain. Cereb Cortex 15:448–459
Reed BT, Sullivan SJ, Tsai G, Coyle T, Esguerra M, Miller RF (2009) The glycine transporter GlyT1 controls N-methyl-d-aspartic acid receptor coagonist occupancy in the mouse retina. Eur J Neurosci 30:2308–2317
Casson RJ (2006) Possible role of excitotoxicity in the pathology of glaucoma. Clin Exp Ophthalmol 34:54–63
Guo L, Salt TE, Maass A, Luong V, Moss SE, Fitzke FW, Cordeiro MF (2006) Assessment of neuroprotective effects of glutamate modulation on glaucoma-related retinal ganglion cell apoptosis in vivo. Invest Ophthalmol Vis Sci 47:626–633
Danysz W, Parson CG (1998) N-Methyl-d-aspartate receptors: physiological significance and possible therapeutic applications. Pharmacol Rev 50:597–66422
Gigler G, Szenasi G, Simo A, Levay G, Harsing LG Jr, Sas K, Vecsei L, Toldi J (2007) Neuroprotective effect of l-kynurenine sulfate administered before focal cerebral ischemia in mice and global cerebral ischemia in gerbils. Eur J Pharmacol 564:116–122
Harsing LG Jr, Gigler G, Moricz K, Agoston M, Marko B, Szabo G, Szenasi G, Juranyi Z (2005) Antiischemic effects of Org-24461 and NFPS, two glycine transporter type-1 inhibitors. Soc Neurosci Abstr 669:16
Oda M, Kure S, Sugawara T, Yamaguchi S, Kojima K, Shinka T, Sato K, Narisawa A, Aoki Y, Matsubara Y, Omae T, Mizoi K, Kinouchi H (2013) Direct correlation between ischemic injury and extracellular glycine concentration in mice with genetically altered activities of the glycine cleavage multienzyme system. Stroke 38:2157–2164
Yao W, Ji F, Chen Z, Zhang N, Ren S-Q, Zhang X-Y, Liu S-Y, Lu W (2012) Glycine exerts dual roles in ischemic injury through distinct mechanisms. Stroke 43:2212–2220
Kertesz S, Szabo G, Udvari S, Levay G, Matyus P, Harsing LG Jr (2013) Temporal alteration of spreading depression by the glycine transporter type-1 inhibitors NFPS and Org-24461 in the chicken retina. Brain Res 1492:1–6
Eyo UB, Miner SA, Ahlers KE, Wu L-J, Dailer ME (2013) P2X7 receptor activation regulates microglial cell death during oxygen–glucose deprivation. Neuropharmacology 73:311–319
Harsing LG Jr, Matyus P (2013) Mechanisms of gycine release, which build up synaptic and extrasynaptic glycine levels: the role of synaptic and non-synaptic glycine transporters. Brain Res Bull 93:110–119
Colleoni S, Jensen AA, Landucci E, Fumagalli E, Conti P, Pinto A, De Amici M, Pellegrini-Giampietro DE, De Michelli C, Mennini T, Gobbi M (2008) Neuroprotective effects of the novel glutamate transporter inhibitor (−)-3-hydroxy-4,5,6,6a-tetrahydro-3aH-pyrollo[3,4-d]-isoxazole-4-carboxylic acid, which preferentially inhibits reverse transport (glutamate release) compared with glutamate reuptake. J Pharmacol Exp Ther 326:646–656
Vizi ES, Harsing LG Jr, Knoll J (1977) Presynaptic inhibition leading to disinhibition of acetylcholine release from interneurons of the caudate nucleus: effect of dopamine, beta-endorphin and d-Ala2-Pro5-enkephalinamide. Neuroscience 2:953–961
Marcaggi P, Hirji N, Attwell D (2005) Release of l-aspartate by reversal of glutamate transporters. Neuropharmacology 49:843–849
Lindsley CW, Zhao Z, Leister WH, O’Brien JA, Lemaire W, Williams DL Jr, Chen T-B, Chang RSL, Burno M, Jacobson MA, Sur C, Kinney GG, Pettibone DJ, Tiller PR, Smith S, Rsou NN, Duggan ME, Conn PJ, Hartman GD (2006) Design, synthesis, and in vivo efficacy of novel glycine transporter-1 (GlyT1) inhibitors derived from a series of [4-phenyl-1-(propyl-sulfonyl)-piperidin-4-yl)-methyl)benzamides. Chem Med Chem 1:807–811
Herdon HJ, Godfrey FM, Brown AM, Coulton S, Evans JR, Cairns WJ (2001) Pharmacological assessment of the role of glycine transporter GlyT-1 in mediating high affinity glycine uptake by rat cerebral cortex and cerebellum synaptosomes. Neuropharmacology 41:88–96
Harsing LG Jr, Gacsalyi I, Szabo G, Schmidt E, Sziray N, Sebban C, Tesolin-Decros B, Matyus P, Egyed A, Spedding M, Levay G (2013) The glycine transporter-1 inhibitors NFPS and Org 24461: a pharmacological study. Pharmacol Biochem Behav 74:811–825
Hardingham GE, Fukunaga Y, Bading H (2002) Extrasynaptic NMDARs oppose synaptic NMDARs by triggering CREB shut-off and cell death pathways. Nat Neurosci 5:405–414
Choo AM, Geddes-Klein DM, Hockemberry A, Scarcella D, Mesfin MN, Singh P, Patel TP, Meaney DF (2012) NR2A and NR2B subunits differentially mediate MAP kinase signaling and mitochondrial morphology following excitotoxic insult. Neurochem Int 60:506–516
Martel M-A, Ryan TJ, Bell KFS, Fowler JH, McMahon A, Al-Mubarak B, Komiyama NH, Horsburgh K, Kind PC, Grant SGN, Wyllie DJA (2012) The subtype of GluN2 C terminal domain determines the response to excitotoxic insults. Neuron 74:543–556
Coyle JT, Puttfarcken P (1993) Oxidative stress, glutamate, and neurodegenerative disorders. Science 262:689–695
Rossi DJ, Oshima T, Attwell D (2000) Glutamate release in severe brain ischemia is mainly by reversed uptake. Nature 403:316–321
Vizi ES (1998) Different temperature dependence of carrier-mediated (cytoplasmic) and stimulus-evoked (exocytotic) release of transmitter: a simple method to separate the two types of release. Neurochem Int 33:359–366
Harsing LG Jr (2013) An overview to glycine transporter type-1 (GlyT-1) inhibitors under evaluation for the treatment of schizophrenia. Drugs Future 38:555–568
Pinto MCX, de Assis Vieira, Lima I, Pessoa da Costa FL, Rosa DV, Mendes-Goulart VA, Resende RR, Romano-Silva MA, Pinheiro de Oliveira AC, Gomez MV, Gomez RS (2015) Glycine transporters type 1 inhibitor promotes brain preconditioning against NMDA-induced excitotoxicity. Neuropharmacology 89:274–281
Napper GA, Pianta MJ, Kalloniatis M (1999) Reduced glutamate uptake by retinal glial cells under ischemic/hypoxic conditions. Vis Neurosci 16:149–158
Bull ND, Barnett NL (2004) Retinal glutamate transporter activity persists under stimulated ischemic conditions. J Neurosci Res 78:590–599
Kallo I, Jekkel C, Hrabovszky E, Juranyi Z, Vida B, Jarasi A, Wilheim T, Harsing LG Jr, Liposits Z (2008) Immunohistochemical and in situ hybridization studies on glycine transporter 1 after transient ischemia in the rat forebrain. Neurochem Int 52:799–808
Rao VLR, Dogan A, Bowen KK, Todd KG, Dempsey RJ (2001) Antisenese knockdown of the glial glutamate transporter GLT-1 exacerbates hippocampal neuronal damage following traumatic injury of the brain. Eur J Neurosci 13:119–128
Mitani A, Tanaka K (2003) Functional changes of glial glutamate transporter GLT-1 during ischemia: an in vivo study in the hippocampal CA1 of normal mice and mutant mice lacking GLT-1. J Neurosci 23:7176–7182
Campiani G, De Angelis M, Armorali S, Fattorusso C, Catalanotti B, Ramunno A, Nacci V, Novellino E, Grewer C, Ionescu D, Rauen T, Griffiths R, Sinclair C, Fumagalli E, Mennini T (2001) A rational approach to the design of selective substrates and potent nontransportable inhibitors of the excitatory amino acid transporter EAAC1 (EAAT3). New glutamate and aspartate analogues as potential neuroprotective agents. J Med Chem 44:2507–2510
Harsing LG Jr, Timar J, Szabo G, Udvari S, Nagy KM, Marko B, Zsilla G, Czompa A, Rapolcsanyi P, Kocsis A, Matyus P (2015) Sarcosine-based glycine transporter type-1 (GlyT-1) inhibitors containing pyridazine moiety: a further search for drugs with potential to influence schizophrenia negative symptoms. Curr Pharm Des 21:2291–2303
Harsing LG Jr, Zsilla G, Matyus P, Nagy KM, Marko B, Zs Gyarmati, Szabo A, Timar J (2012) Interactions between glycine transporter type 1 (GlyT-1) and some inhibitor molecules. Acta Physiol Hung 99:1–17
Sperlagh B, Szabo G, Erdelyi F, Baranyi M, Vizi ES (2003) Homo- and heteroexchange of adenine nucleotides and nucleosides in rat hippocampal slices by the nucleoside transport system. Br J Pharmacol 139:623–633
Miller GM (2011) The emerging role of trace amine associated receptor 1 in the functional regulation of monoamine transporters and dopaminergic activity. J Neurochem 116:164–176
Scholze P, Zwach J, Kattinger A, Pifl C, Singer EA, Sitte HH (2000) Transport-mediated release: a superfusion study on human embryonic kidney cells stably expressing the human serotonin transporter. J Pharmacol Exp Ther 293:870–878
Vizi ES, Sperlagh B (1999) Separation of carrier mediated and vesicular release of GABA from rat brain slices. Neurochem Int 34:407–413
Wolburg H (1975) Time- and dose-dependent influence of ouabain on the ultrastructure of optic neurones. Cell Tissue Res 164:503–517
Stuermer CA, Niepenberg A, Wolburg H (1985) Aberrant axonal paths in regenerated goldfish retina and tectum opticum following intraocular injection of ouabain. Neurosci Lett 58:333–338
Milusheva E, Sperlagh B, Shikova L, Baranyi M, Tretter L, Adam-Vizi V, Vizi ES (2003) Non-synaptic release of [3H]noradrenaline in response to oxidative stress combined with mitochondrial dysfunction in rat hippocampal slices. Neuroscience 120:771–778
Bissonnette P, Gagne H, Blais A, Berteloot A (1996) 2-Deoxyglucose transport and metabolism in Caco-2 cells. Am J Physiol 270:G153–G162
Rojas JC, Saavedra JA, Gonzales-Lima F (2008) Neuroprotective effects of memantine in a mouse model of retinal degeneration induced by rotenone. Brain Res 1215:208–217
Kalbaugh TL, Zhang J, Diamond JS (2009) Coagonist release modulates NMDA receptor subtype contributions at synaptic inputs to retinal ganglion cells. J Neurosci 29:1469–1479
Liu Y, Wong TP, Aarts M, Rooyakkers A, Liu L, Lai TW, Wu DC, Lu J, Tymianski M, Craig AM, Wang YT (2007) NMDA receptor subunits have differential roles in mediating excitotoxic neuronal death in vitro and in vivo. J Neurosci 27:2846–2857
Papouin T, Ladepeche L, Ruel J, Sacchi S, Labasque M, Hanini M, Groc L, Pollegioni L, Mothet J-P, Oliet SHR (2012) Synaptic and extrasynaptic NMDA receptors are gated by different endogenous coagonists. Cell 150:633–646
Hardingham GE, Bading H (2003) The Yin and Yang of NMDA receptor signalling. Trends Neurosci 26:81–89
Parsons MP, Raymond LA (2014) Extrasynaptic NMDA receptor involvement in central nervous system disorders. Neurons 82:279–293
Pena-Rangel T, Riesgo-Escovar JR, Sancez-Chavez G, Salcedo R (2008) Glycine transporters (glycine transporter 1 and glycine transporter 2) are expressed in retina. NeuroReport 19:1295–1299
Okamoto M, S-i Akanuma, Tachikawa M, K-i Hosoya (2009) Characteristics of glycine transport across the inner blood–retinal barrier. Neurochem Int 55:789–795
Sullivan SJ, Miller RF (2010) AMPA receptor mediated d-serine release from retinal glial cells. J Neurochem 115:1681–1689
Gouix E, Leveille F, Nicole O, Melon C, Had-Aissouni L, Buisson A (2009) Reverse glial glutamate uptake triggers neuronal cell death through extrasynaptic NMDA receptor activation. Mol Cell Neurosci 40:463–473
Wakabayashi Y, Yagihashi T, Kezuka J, Muramatsu D, Ususi M, Iwasaki T (2006) Glutamate levels in aqueous humour of patients with retinal artery occlusion. Retina 26:432–436
Harsing LG Jr, Hannuska A, Matyus P (2011) Reversal of hypoxia-induced [3H]glycine release from isolated retina by glycine transporter type-1 (GlyT-1 inhibitors. Soc Neurosci Abstr 62:10
Harsing LG Jr, Hanuska A, Varga A, Matyus P, Szenasi G, Kertesz S, Albert M, Levay G (2012) Glycinergic neurotransmission in normal and hypoxic conditions: the role of glycine transporters. Soc Neurosci Abstr 536:26
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors claim no conflicts of interest.
Additional information
Special Issue: 40th year of neurochemical research.
Rights and permissions
About this article
Cite this article
Hanuska, A., Szénási, G., Albert, M. et al. Some Operational Characteristics of Glycine Release in Rat Retina: The Role of Reverse Mode Operation of Glycine Transporter Type-1 (GlyT-1) in Ischemic Conditions. Neurochem Res 41, 73–85 (2016). https://doi.org/10.1007/s11064-015-1713-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-015-1713-z