Abstract
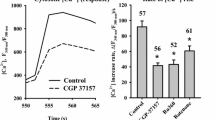
We have previously shown that synaptic transmission fails in cultured neurons in the presence of lactate as the sole substrate. Thus, to test the hypothesis that the failure of synaptic transmission is a consequence of insufficient energy supply, ATP levels were monitored employing the ATP biosensor Ateam1.03YEMK. While inducing synaptic activity by subjecting cultured neurons to two 30 s pulses of NMDA (30 µM) with a 4 min interval, changes in relative ATP levels were measured in the presence of lactate (1 mM), glucose (2.5 mM) or the combination of the two. ATP levels reversibly declined following NMDA-induced neurotransmission activity, as indicated by a reversible 10–20 % decrease in the response of the biosensor. The responses were absent when the NMDA receptor antagonist memantine was present. In the presence of lactate alone, the ATP response dropped significantly more than in the presence of glucose following the 2nd pulse of NMDA (approx. 10 vs. 20 %). Further, cytosolic Ca2+ homeostasis during NMDA-induced synaptic transmission is partially inhibited by verapamil indicating that voltage-gated Ca2+ channels are activated. Lastly, we showed that cytosolic Ca2+ homeostasis is supported equally well by both glucose and lactate, and that a pulse of NMDA causes accumulation of Ca2+ in the mitochondrial matrix. In summary, we have shown that ATP homeostasis during neurotransmission activity in cultured neurons is supported by both glucose and lactate. However, ATP homeostasis seems to be negatively affected by the presence of lactate alone, suggesting that glucose is needed to support neuronal energy metabolism during activation.





Similar content being viewed by others
References
Howarth C, Gleeson P, Attwell D (2012) Updated energy budgets for neural computation in the neocortex and cerebellum. J Cereb Blood Flow Metab 32:1222–1232
McKenna M, Dienel GA, Sonnewald U, Waagepetersen HS, Schousboe A (2012) Energy metabolism of the brain. In: Brady ST, Siegel GJ, Albers RW, Price DI (eds) Basic neurochemistry, 8th edn. Academic Press, Waltham, pp 200–231
Pellerin L, Magistretti PJ (1994) Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proc Natl Acad Sci USA 91:10625–10629
Dienel GA (2012) Brain lactate metabolism: the discoveries and the controversies. J Cereb Blood Flow Metab 32:1107–1138
Dienel GA (2013) Astrocytic energetics during excitatory neurotransmission: what are contributions of glutamate oxidation and glycolysis? Neurochem Int 63:244–258
Mangia S, DiNuzzo M, Giove F, Carruthers A, Simpson IA, Vannucci SJ (2011) Response to ‘comment on recent modeling studies of astrocyte-neuron metabolic interactions’: much ado about nothing. J Cereb Blood Flow Metab 31:1346–1353
Mergenthaler P, Lindauer U, Dienel GA, Meisel A (2013) Sugar for the brain: the role of glucose in physiological and pathological brain function. Trend Neurosci 36:587–597
Bak LK, Schousboe A, Sonnewald U, Waagepetersen HS (2006) Glucose is necessary to maintain neurotransmitter homeostasis during synaptic activity in cultured glutamatergic neurons. J Cereb Blood Flow Metab 26:1285–1297
Imamura H, Nhat KP, Togawa H, Saito K, Iino R, Kato-Yamada Y, Nagai T, Noji H (2009) Visualization of ATP levels inside single living cells with fluorescence resonance energy transfer-based genetically encoded indicators. Proc Natl Acad Sci USA 106:15651–15656
Schousboe A, Meier E, Drejer J, Hertz L (1989) Preparation of primary cultures of mouse (rat) cerebellar granule cells. In: Shahar A, de Vellis J, Vernadakis A, Haber B (eds) A dissection and tissue culture manual for the nervous system. Alan R. Liss, New York, pp 183–186
Zhao Y, Araki S, Wu J, Teramoto T, Chang YF, Nakano M, Abdelfattah AS, Fujiwara M, Ishihara T, Nagai T, Campbell RE (2011) An expanded palette of genetically encoded Ca(2)(+) indicators. Science 333:1888–1891
Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez JY, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A (2012) Fiji: an open-source platform for biological-image analysis. Nat Methods 9:676–682
Yang X, Boehm JS, Yang X, Salehi-Ashtiani K, Hao T, Shen Y, Lubonja R, Thomas SR, Alkan O, Bhimdi T, Green TM, Johannessen CM, Silver SJ, Nguyen C, Murray RR, Hieronymus H, Balcha D, Fan C, Lin C, Ghamsari L, Vidal M, Hahn WC, Hill DE, Root DE (2011) A public genome-scale lentiviral expression library of human ORFs. Nat Methods 8:659–661
Stewart SA, Dykxhoorn DM, Palliser D, Mizuno H, Yu EY, An DS, Sabatini DM, Chen IS, Hahn WC, Sharp PA, Weinberg RA, Novina CD (2003) Lentivirus-delivered stable gene silencing by RNAi in primary cells. RNA 9:493–501
Bak LK, Schousboe A, Waagepetersen HS (2003) Characterization of depolarization-coupled release of glutamate from cultured mouse cerebellar granule cells using DL-threo-beta-benzyloxyaspartate (DL-TBOA) to distinguish between the vesicular and cytoplasmic pools. Neurochem Int 43:417–424
Mobius HJ, Stoffler A, Graham SM (2004) Memantine hydrochloride: pharmacological and clinical profile. Drugs Today 40:685–695
Carboni E, Wojcik WJ (1988) Dihydropyridine binding sites regulate calcium influx through specific voltage-sensitive calcium channels in cerebellar granule cells. J Neurochem 50:1279–1286
Bak LK, Walls AB, Schousboe A, Ring A, Sonnewald U, Waagepetersen HS (2009) Neuronal glucose but not lactate utilization is positively correlated with NMDA-induced neurotransmission and fluctuations in cytosolic Ca2+ levels. J Neurochem 109:87–93
Contreras L, Satrustegui J (2009) Calcium signaling in brain mitochondria: interplay of malate aspartate NADH shuttle and calcium uniporter/mitochondrial dehydrogenase pathways. J Biol Chem 284:7091–7099
O’Brien J, Kla KM, Hopkins IB, Malecki EA, McKenna MC (2007) Kinetic parameters and lactate dehydrogenase isozyme activities support possible lactate utilization by neurons. Neurochem Res 32:597–607
Quistorff B, Grunnet N (2011) The isoenzyme pattern of LDH does not play a physiological role; except perhaps during fast transitions in energy metabolism. Aging 3:457–460
Bak LK, Obel LF, Walls AB, Schousboe A, Faek SAA, Jajo FS, Waagepetersen HS (2012) Novel model of neuronal bioenergetics: postsynaptic utilization of glucose but not lactate correlates positively with Ca2+ signalling in cultured mouse glutamatergic neurons. ASN Neuro 4(3). pii: e00083
Bouzier-Sore AK, Voisin P, Canioni P, Magistretti PJ, Pellerin L (2003) Lactate is a preferential oxidative energy substrate over glucose for neurons in culture. J Cereb Blood Flow Metab 23:1298–1306
Waagepetersen HS, Bakken IJ, Larsson OM, Sonnewald U, Schousboe A (1998) Comparison of lactate and glucose metabolism in cultured neocortical neurons and astrocytes using 13C-NMR spectroscopy. Dev Neurosci 20:310–320
Waagepetersen HS, Bakken IJ, Larsson OM, Sonnewald U, Schousboe A (1998) Metabolism of lactate in cultured GABAergic neurons studied by 13C nuclear magnetic resonance spectroscopy. J Cereb Blood Flow Metab 18:109–117
Hertz L, Gibbs ME, Dienel GA (2014) Fluxes of lactate into, from, and among gap junction-coupled astrocytes and their interaction with noradrenaline. Front Neurosci 8:261
Fox PT, Raichle ME, Mintun MA, Dence C (1988) Nonoxidative glucose consumption during focal physiologic neural activity. Science 241:462–464
Galow LV, Schneider J, Lewen A, Ta TT, Papageorgiou IE, Kann O (2014) Energy substrates that fuel fast neuronal network oscillations. Front Neurosci 8:398
Lundgaard I, Li B, Xie L, Kang H, Sanggaard S, Haswell JD, Sun W, Goldman S, Blekot S, Nielsen M, Takano T, Deane R, Nedergaard M (2015) Direct neuronal glucose uptake heralds activity-dependent increases in cerebral metabolism. Nat Commun 6:6807
Dinuzzo M, Mangia S, Maraviglia B, Giove F (2012) The role of astrocytic glycogen in supporting the energetics of neuronal activity. Neurochem Res 37(11):2432–2438
Patel AB, Lai JC, Chowdhury GM, Hyder F, Rothman DL, Shulman RG, Behar KL (2014) Direct evidence for activity-dependent glucose phosphorylation in neurons with implications for the astrocyte-to-neuron lactate shuttle. Proc Natl Acad Sci USA 111(14):5385–5390
Jakoby P, Schmidt E, Ruminot I, Gutiérrez R, Barros LF, Deitmer JW (2014) Higher transport and metabolism of glucose in astrocytes compared with neurons: a multiphoton study of hippocampal and cerebellar tissue slices. Cereb Cortex 24(1):222–231
Barros LF, Courjaret R, Jakoby P, Loaiza A, Lohr C, Deitmer JW (2009) Preferential transport and metabolism of glucose in Bergmann glia over Purkinje cells: a multiphoton study of cerebellar slices. Glia 57(9):962–970
Chuquet J, Quilichini P, Nimchinsky EA, Buzsáki G (2010) Predominant enhancement of glucose uptake in astrocytes versus neurons during activation of the somatosensory cortex. J Neurosci 30(45):15298–15303
DiNuzzo M, Giove F, Maraviglia B, Mangia S (2013) Glucose metabolism down-regulates the uptake of 6-(N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino)-2-deoxyglucose (6-NBDG) mediated by glucose transporter 1 isoform (GLUT1): theory and simulations using the symmetric four-state carrier model. J Neurochem 125(2):236–246
Lauritzen KH, Morland C, Puchades M, Holm-Hansen S, Hagelin EM, Lauritzen F, Attramadal H, Storm-Mathisen J, Gjedde A, Bergersen LH (2014) Lactate receptor sites link neurotransmission, neurovascular coupling, and brain energy metabolism. Cereb Cortex 24:2784–2795
Acknowledgments
The authors would like to thank Dr. Judith Stegmüller, Max-Planck-Institute for Experimental Medicine, Göttingen, Germany for instrumental advice regarding transfection of CCNs as well as Dr. Marlen Michaelis, Carl-Ludwig-Institute for Physiology, Leipzig, Germany for initial help with the fura-2 imaging setup. The Danish Medical Research Council (Grant No. 11-108039) is cordially acknowledged for providing financial support to H.S.W., and we are grateful for the support of the Hørslev Foundation to S.C.L.
Author information
Authors and Affiliations
Corresponding author
Additional information
Special Issue: In honor of Dr. Gerald Dienel.
Rights and permissions
About this article
Cite this article
Lange, S.C., Winkler, U., Andresen, L. et al. Dynamic Changes in Cytosolic ATP Levels in Cultured Glutamatergic Neurons During NMDA-Induced Synaptic Activity Supported by Glucose or Lactate. Neurochem Res 40, 2517–2526 (2015). https://doi.org/10.1007/s11064-015-1651-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-015-1651-9