Abstract
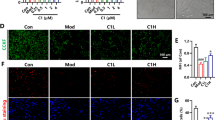
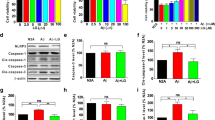
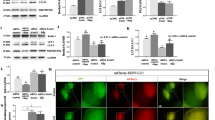
Alzheimer’s disease (AD) is associated with the inflammatory response in response to amyloid β-peptide (Aβ). Previous studies have suggested that paeoniflorin (PF) shows anti-inflammatory and neuroprotective effects in inflammation-related diseases. However, the impacts of PF on AD have not been investigated. In the present study, we showed that a 4-week treatment with PF could significantly inhibit Aβ burden, Aβ-induced over activation of astrocytes and microglia, downregulation of proinflammatory cytokines, and upregulation of anti-inflammatory cytokines in the brain. In addition, we demonstrated that chronic treatment with PF inhibited the activation of glycogen synthase kinase 3β (GSK-3β) and reversed neuroinflammtory-induced activation of nuclear factor-kappa B (NF-κB) signaling pathways. Moreover, PF exerted inhibitory effects on NALP3 inflammasome, caspase-1, and IL-1β. Collectively, in the present study, we demonstrated that PF exhibits neuroprotective effects in amyloid precursor protein (APP) and presenilin 1 (PS1) double-transgenic (APP/PS1) mice via inhibiting neuroinflammation mediated by the GSK-3β and NF-κB signaling pathways and nucleotide-binding domain-like receptor protein 3 inflammasome. Thus, these results suggest that PF might be useful to intervene in development or progression of neurodegeneration in AD through its anti-inflammatory and anti-amyloidogenic effects.






Similar content being viewed by others
References
Cummings JL, Cole G (2002) Alzheimer disease. JAMA 287:2335–2338
Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, Cooper NR, Eikelenboom P, Emmerling M, Fiebich BL, Finch CE, Frautschy S, Griffin WS, Hampel H, Hull M, Landreth G, Lue L, Mrak R, Mackenzie IR, McGeer PL, O’Banion MK, Pachter J, Pasinetti G, Plata-Salaman C, Rogers J, Rydel R, Shen Y, Streit W, Strohmeyer R, Tooyoma I, Van Muiswinkel FL, Veerhuis R, Walker D, Webster S, Wegrzyniak B, Wenk G, Wyss-Coray T (2000) Inflammation and Alzheimer’s disease. Neurobiol Aging 21:383–421
Fan R, Xu F, Previti ML, Davis J, Grande AM, Robinson JK, Van Nostrand WE (2007) Minocycline reduces microglial activation and improves behavioral deficits in a transgenic model of cerebral microvascular amyloid. J Neurosci 27:3057–3063
Ly PT, Wu Y, Zou H, Wang R, Zhou W, Kinoshita A, Zhang M, Yang Y, Cai F, Woodgett J, Song W (2013) Inhibition of GSK3beta-mediated BACE1 expression reduces Alzheimer-associated phenotypes. J Clin Investig 123:224–235
Yuskaitis CJ, Jope RS (2009) Glycogen synthase kinase-3 regulates microglial migration, inflammation, and inflammation-induced neurotoxicity. Cell Signal 21:264–273
Beurel E, Jope RS (2009) Lipopolysaccharide-induced interleukin-6 production is controlled by glycogen synthase kinase-3 and STAT3 in the brain. J Neuroinflammation 6:9
Salminen A, Ojala J, Suuronen T, Kaarniranta K, Kauppinen A (2008) Amyloid-beta oligomers set fire to inflammasomes and induce Alzheimer’s pathology. J Cell Mol Med 12:2255–2262
Halle A, Hornung V, Petzold GC, Stewart CR, Monks BG, Reinheckel T, Fitzgerald KA, Latz E, Moore KJ, Golenbock DT (2008) The NALP3 inflammasome is involved in the innate immune response to amyloid-beta. Nat Immunol 9:857–865
Palop JJ, Chin J, Roberson ED, Wang J, Thwin MT, Bien-Ly N, Yoo J, Ho KO, Yu GQ, Kreitzer A, Finkbeiner S, Noebels JL, Mucke L (2007) Aberrant excitatory neuronal activity and compensatory remodeling of inhibitory hippocampal circuits in mouse models of Alzheimer’s disease. Neuron 55:697–711
Bauernfeind FG, Horvath G, Stutz A, Alnemri ES, MacDonald K, Speert D, Fernandes-Alnemri T, Wu J, Monks BG, Fitzgerald KA, Hornung V, Latz E (2009) Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J Immunol 183:787–791
Heneka MT, Kummer MP, Stutz A, Delekate A, Schwartz S, Vieira-Saecker A, Griep A, Axt D, Remus A, Tzeng TC, Gelpi E, Halle A, Korte M, Latz E, Golenbock DT (2013) NLRP3 is activated in Alzheimer’s disease and contributes to pathology in APP/PS1 mice. Nature 493:674–678
Wu SH, Wu DG, Chen YW (2010) Chemical constituents and bioactivities of plants from the genus Paeonia. Chem Biodivers 7:90–104
Nizamutdinova IT, Jin YC, Kim JS, Yean MH, Kang SS, Kim YS, Lee JH, Seo HG, Kim HJ, Chang KC (2008) Paeonol and paeoniflorin, the main active principles of Paeonia albiflora, protect the heart from myocardial ischemia/reperfusion injury in rats. Planta Med 74:14–18
Guo RB, Wang GF, Zhao AP, Gu J, Sun XL, Hu G (2012) Paeoniflorin protects against ischemia-induced brain damages in rats via inhibiting MAPKs/NF-kappaB-mediated inflammatory responses. PLoS ONE 7:e49701
Jiang D, Chen Y, Hou X, Xu J, Mu X, Chen W (2011) Influence of Paeonia lactiflora roots extract on cAMP-phosphodiesterase activity and related anti-inflammatory action. J Ethnopharmacol 137:914–920
Wang D, Wong HK, Feng YB, Zhang ZJ (2013) Paeoniflorin, a natural neuroprotective agent, modulates multiple anti-apoptotic and pro-apoptotic pathways in differentiated PC12 cells. Cell Mol Neurobiol 33:521–529
Zhong SZ, Ge QH, Li Q, Qu R, Ma SP (2009) Peoniflorin attentuates Abeta(1–42)-mediated neurotoxicity by regulating calcium homeostasis and ameliorating oxidative stress in hippocampus of rats. J Neurol Sci 280:71–78
Liu HQ, Zhang WY, Luo XT, Ye Y, Zhu XZ (2006) Paeoniflorin attenuates neuroinflammation and dopaminergic neurodegeneration in the MPTP model of Parkinson’s disease by activation of adenosine A1 receptor. Br J Pharmacol 148:314–325
Liu DZ, Xie KQ, Ji XQ, Ye Y, Jiang CL, Zhu XZ (2005) Neuroprotective effect of paeoniflorin on cerebral ischemic rat by activating adenosine A1 receptor in a manner different from its classical agonists. Br J Pharmacol 146:604–611
Vorhees CV, Williams MT (2006) Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc 1:848–858
Zhang M-Y, Zheng C-Y, Zou M-M, Zhu J-W, Zhang Y, Wang J, Liu C-F, Li Q-F, Xiao Z-C, Li S (2014) Lamotrigine attenuates deficits in synaptic plasticity and accumulation of amyloid plaques in APP/PS1 transgenic mice. Neurobiol Aging 35:2713–2725
Mrak RE, Griffin WS (2005) Glia and their cytokines in progression of neurodegeneration. Neurobiol Aging 26:349–354
Zhang R, Miller RG, Madison C, Jin X, Honrada R, Harris W, Katz J, Forshew DA, McGrath MS (2013) Systemic immune system alterations in early stages of Alzheimer’s disease. J Neuroimmunol 256:38–42
Nunes AF, Amaral JD, Lo AC, Fonseca MB, Viana RJ, Callaerts-Vegh Z, D’Hooge R, Rodrigues CM (2012) TUDCA, a bile acid, attenuates amyloid precursor protein processing and amyloid-beta deposition in APP/PS1 mice. Mol Neurobiol 45:440–454
Ding Y, Qiao A, Wang Z, Goodwin JS, Lee ES, Block ML, Allsbrook M, McDonald MP, Fan GH (2008) Retinoic acid attenuates beta-amyloid deposition and rescues memory deficits in an Alzheimer’s disease transgenic mouse model. J Neurosci 28:11622–11634
He P, Zhong Z, Lindholm K, Berning L, Lee W, Lemere C, Staufenbiel M, Li R, Shen Y (2007) Deletion of tumor necrosis factor death receptor inhibits amyloid beta generation and prevents learning and memory deficits in Alzheimer’s mice. J Cell Biol 178:829–841
Shi JQ, Zhang CC, Sun XL, Cheng XX, Wang JB, Zhang YD, Xu J, Zou HQ (2013) Antimalarial drug artemisinin extenuates amyloidogenesis and neuroinflammation in APPswe/PS1dE9 transgenic mice via inhibition of nuclear factor-kappaB and NLRP3 inflammasome activation. CNS Neurosci Ther 19:262–268
Wyss-Coray T, Rogers J (2012) Inflammation in Alzheimer disease—a brief review of the basic science and clinical literature. Cold Spring Harbor Perspect Med 2:a006346
Lin GH, Lee YJ, Choi DY, Han SB, Jung JK, Hwang BY, Moon DC, Kim Y, Lee MK, Oh KW, Jeong HS, Leem JY, Shin HK, Lee JH, Hong JT (2012) Anti-amyloidogenic effect of thiacremonone through anti-inflamation in vitro and in vivo models. JAD 29:659–676
Lee YJ, Choi DY, Choi IS, Kim KH, Kim YH, Kim HM, Lee K, Cho WG, Jung JK, Han SB, Han JY, Nam SY, Yun YW, Jeong JH, Oh KW, Hong JT (2012) Inhibitory effect of 4-O-methylhonokiol on lipopolysaccharide-induced neuroinflammation, amyloidogenesis and memory impairment via inhibition of nuclear factor-kappaB in vitro and in vivo models. J Neuroinflam. 9:35
Choi DY, Lee JW, Peng J, Lee YJ, Han JY, Lee YH, Choi IS, Han SB, Jung JK, Lee WS, Lee SH, Kwon BM, Oh KW, Hong JT (2012) Obovatol improves cognitive functions in animal models for Alzheimer’s disease. J Neurochem 120:1048–1059
Puli L, Pomeshchik Y, Olas K, Malm T, Koistinaho J, Tanila H (2012) Effects of human intravenous immunoglobulin on amyloid pathology and neuroinflammation in a mouse model of Alzheimer’s disease. J Neuroinflam. 9:105
Ruan L, Kang Z, Pei G, Le Y (2009) Amyloid deposition and inflammation in APPswe/PS1dE9 mouse model of Alzheimer’s disease. Curr Alzheimer Res 6:531–540
Takada Y, Fang X, Jamaluddin MS, Boyd DD, Aggarwal BB (2004) Genetic deletion of glycogen synthase kinase-3beta abrogates activation of IkappaBalpha kinase, JNK, Akt, and p44/p42 MAPK but potentiates apoptosis induced by tumor necrosis factor. J Biol Chem 279:39541–39554
Steinbrecher KA, Wilson W 3rd, Cogswell PC, Baldwin AS (2005) Glycogen synthase kinase 3beta functions to specify gene-specific NF-kappaB-dependent transcription. Mole Cell Biol 25:8444–8455
Paris D, Patel N, Quadros A, Linan M, Bakshi P, Ait-Ghezala G, Mullan M (2007) Inhibition of Abeta production by NF-kappaB inhibitors. Neurosci Lett 415:11–16
Acknowledgments
This study was supported by funding from the “National Natural Science Foundation of guangdong’’ (Grant No. 32215050) and “Scientific research and innovation project of Jinan University” (Grant No. 21615336). This work was also supported by the “Scientific research and innovation project of Luoyang”(Grant No. 150415), the “Natural Scientific Research funds of China” (No. 81301116) and “China Postdoctoral Science Foundation”(Grant No. 2012M521922).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Zhang, HR., Peng, JH., Cheng, XB. et al. Paeoniflorin Atttenuates Amyloidogenesis and the Inflammatory Responses in a Transgenic Mouse Model of Alzheimer’s Disease. Neurochem Res 40, 1583–1592 (2015). https://doi.org/10.1007/s11064-015-1632-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-015-1632-z