Abstract
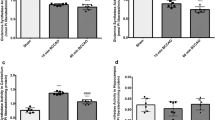
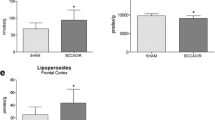
Brain lipid metabolism was studied in rats following permanent bilateral common carotid artery ligation (BCCL), a model for chronic cerebral hypoperfusion. Unesterified (free) fatty acids (uFA) and acyl-CoA concentrations were measured 6 h, 24 h, and 7 days after BCCL or sham surgery, in high energy-microwaved brain. In BCCL compared to sham rats, cytosolic phospholipase A2 (cPLA2) immunoreactivity in piriform cortex, and concentrations of total uFA and arachidonoyl-CoA, an intermediate for arachidonic acid reincorporation into phospholipids, were increased only at 6 h. At 24 h, immunoreactivity for secretory phospholipase A2 (sPLA2), which may regulate blood flow, was increased near cortical and hippocampal blood vessels. BCCL did not affect levels of brain IB4+ microglia, glial fibrillary acidic protein (GFAP)+ astrocytes, cyclooxygenase-2 (COX-2) immunoreactivity at any time, but increased cPLA2 immunoreactivity in one region at 6 h. Thus, BCCL affected brain lipid metabolism transiently, likely because of compensatory sPLA2-mediated vasodilation, without producing evidence of neuroinflammation.



Similar content being viewed by others
Abbreviations
- ARA:
-
Arachidonic acid
- BCCL:
-
Bilateral common carotid artery ligation
- cPLA2 :
-
Cytoplasmic phospholipase A2
- sPLA2 :
-
Secretory phospholipase A2
- CBF:
-
Cerebral blood flow
- COX-2:
-
Cyclooxygenase 2
- HRP:
-
Horseradish peroxidase
- IB4 :
-
Isolectin B4
- GFAP:
-
Glial fibrillary acidic protein
- uFA:
-
Unesterified fatty acid
- PGE2 :
-
Prostaglandin E2
References
de la Torre JC (2006) How do heart disease and stroke become risk factors for Alzheimer’s disease? Neurol Res 28:637–644
Kalaria RN (2000) The role of cerebral ischemia in Alzheimer’s disease. Neurobiol Aging 21:321–330
Siesjö BK (1978) Brain energy metabolism. Wiley, Chichester, pp 1–607
Kalaria RN, Kenny RA, Ballard CG, Perry R, Ince P, Polvikoski T (2004) Towards defining the neuropathological substrates of vascular dementia. J Neurol Sci 226:75–80
Napoli C, Palinski W (2005) Neurodegenerative diseases: insights into pathogenic mechanisms from atherosclerosis. Neurobiol Aging 26:293–302
Hachinski V, Munoz D (2000) Vascular factors in cognitive impairment–where are we now? Ann NY Acad Sci 903:1–5
Esposito G, Giovacchini G, Liow JS, Bhattacharjee AK, Greenstein D, Schapiro M, Hallett M, Herscovitch P, Eckelman WC, Carson RE, Rapoport SI (2008) Imaging neuroinflammation in Alzheimer’s disease with radiolabeled arachidonic acid and PET. J Nucl Med 49:1414–1421
Roman GC (2005) Vascular dementia prevention: a risk factor analysis. Cerebrovasc Dis 20(Suppl 2):91–100
Andin U, Gustafson L, Passant U, Brun A (2005) A clinico-pathological study of heart and brain lesions in vascular dementia. Dement Geriatr Cogn Disord 19:222–228
Scheel P, Puls I, Becker G, Schoning M (1999) Volume reduction in cerebral blood flow in patients with vascular dementia. Lancet 354:2137
Komatani A, Yamaguchi K, Sugai Y, Takanashi T, Kera M, Shinohara M, Kawakatsu S (1988) Assessment of demented patients by dynamic SPECT of inhaled xenon-133. J Nucl Med 29:1621–1626
Niedermeyer E (2006) Alzheimer disease: caused by primary deficiency of the cerebral blood flow. Clin EEG Neurosci 37:175–177
Swan JH, Evans MC, Meldrum BS (1988) Long-term development of selective neuronal loss and the mechanism of protection by 2-amino-7-phosphonoheptanoate in a rat model of incomplete forebrain ischaemia. J Cereb Blood Flow Metab 8:64–78
Farkas E, Luiten PG, Bari F (2007) Permanent, bilateral common carotid artery occlusion in the rat: a model for chronic cerebral hypoperfusion-related neurodegenerative diseases. Brain Res Rev 54:162–180
Kunimatsu T, Asai S, Kanematsu K, Kohno T, Misaki T, Ishikawa K (2001) Effects of glutamate receptor agonist on extracellular glutamate dynamics during moderate cerebral ischemia. Brain Res 923:178–186
Otori T, Katsumata T, Katayama Y, Terashi A (1997) Measurement of regional cerebral blood flow and glucose utilization in rat brain under chronic hypoperfusion conditions following bilateral carotid artery occlusion. Analyzed by autoradiographical methods. Nippon Ika Daigaku Zasshi 64:428–439
Institoris A, Farkas E, Berczi S, Sule Z, Bari F (2007) Effects of cyclooxygenase (COX) inhibition on memory impairment and hippocampal damage in the early period of cerebral hypoperfusion in rats. Eur J Pharmacol 574:29–38
de la Torre JC, Cada A, Nelson N, Davis G, Sutherland RJ, Gonzalez-Lima F (1997) Reduced cytochrome oxidase and memory dysfunction after chronic brain ischemia in aged rats. Neurosci Lett 223:165–168
Ni JW, Matsumoto K, Li HB, Murakami Y, Watanabe H (1995) Neuronal damage and decrease of central acetylcholine level following permanent occlusion of bilateral common carotid arteries in rat. Brain Res 673:290–296
Nanri M, Miyake H, Murakami Y, Matsumoto K, Watanabe H (1998) Chronic cerebral hypoperfusion-induced neuropathological changes in rats. Nihon Shinkei Seishin Yakurigaku Zasshi 18:181–188
Tanaka K, Wada-Tanaka N, Miyazaki I, Nomura M, Ogawa N (2002) Chronic cerebral hypoperfusion induces striatal alterations due to the transient increase of NO production and the depression of glutathione content. Neurochem Res 27:331–336
Otori T, Katsumata T, Muramatsu H, Kashiwagi F, Katayama Y, Terashi A (2003) Long-term measurement of cerebral blood flow and metabolism in a rat chronic hypoperfusion model. Clin Exp Pharmacol Physiol 30:266–272
Liu HX, Zhang JJ, Zheng P, Zhang Y (2005) Altered expression of MAP-2, GAP-43, and synaptophysin in the hippocampus of rats with chronic cerebral hypoperfusion correlates with cognitive impairment. Brain Res Mol Brain Res 139:169–177
Farkas B, Tantos A, Schlett K, Vilagi I, Friedrich P (2004) Ischemia-induced increase in long-term potentiation is warded off by specific calpain inhibitor PD150606. Brain Res 1024:150–158
Schmidt-Kastner R, Aguirre-Chen C, Saul I, Yick L, Hamasaki D, Busto R, Ginsberg MD (2005) Astrocytes react to oligemia in the forebrain induced by chronic bilateral common carotid artery occlusion in rats. Brain Res 1052:28–39
Liu D, Wu L, Breyer R, Mattson MP, Andreasson K (2005) Neuroprotection by the PGE2 EP2 receptor in permanent focal cerebral ischemia. Ann Neurol 57:758–761
Lu J, Tong XY, Wang XL (2002) Altered gene expression of Na+/Ca2+ exchanger isoforms NCX1, NCX2 and NCX3 in chronic ischemic rat brain. Neurosci Lett 332:21–24
Purdon AD, Rapoport SI (2007) Energy consumption by phospholipid metabolism in mammalian brain. In: Gibson G, Dienel G (eds) Neural energy utilization: handbook of neurochemistry and molecular biology, vol 16. Springer, New York, pp 401–427
Rabin O, Chang MC, Grange E, Bell J, Rapoport SI, Deutsch J, Purdon AD (1998) Selective acceleration of arachidonic acid reincorporation into brain membrane phospholipid following transient ischemia in awake gerbil. J Neurochem 70:325–334
Rabin O, Deutsch J, Grange E, Pettigrew KD, Chang MC, Rapoport SI, Purdon AD (1997) Changes in cerebral acyl-CoA concentrations following ischemia-reperfusion in awake gerbils. J Neurochem 68:2111–2118
Deutsch J, Rapoport SI, Purdon AD (1997) Relation between free fatty acid and acyl-CoA concentrations in rat brain following decapitation. Neurochem Res 22:759–765
Kuwashima J, Nakamura K, Fujitani B, Kadokawa T, Yoshida K, Shimizu M (1978) Relationship between cerebral energy failure and free fatty acid accumulation following prolonged brain ischemia. Jpn J Pharmacol 28:277–287
Murphy EJ (2010) Brain fixation for analysis of brain lipid-mediators of signal transduction and brain eicosanoids requires head-focused microwave irradiation: an historical perspective. Prostaglandins Other Lipid Mediat 91:63–67
Robinson PJ, Noronha J, DeGeorge JJ, Freed LM, Nariai T, Rapoport SI (1992) A quantitative method for measuring regional in vivo fatty-acid incorporation into and turnover within brain phospholipids: review and critical analysis. Brain Res Rev 17:187–214
Sun GY, MacQuarrie RA (1989) Deacylation-reacylation of arachidonoyl groups in cerebral phospholipids. Ann NY Acad Sci 559:37–55
Ong WY, Sandhya TL, Horrocks LA, Farooqui AA (1999) Distribution of cytoplasmic phospholipase A2 in the normal rat brain. J Hirnforsch 39:391–400
Pardue S, Rapoport SI, Bosetti F (2003) Co-localization of cytosolic phospholipase A2 and cyclooxygenase-2 in Rhesus monkey cerebellum. Brain Res Mol Brain Res 116:106–114
Shimizu T, Wolfe LS (1990) Arachidonic acid cascade and signal transduction. J Neurochem 55:1–15
Bazán NG (1989) Arachidonic acid in the modulation of excitable membrane function and at the onset of brain damage. Ann NY Acad Sci 559:1–16
Folch J, Lees M, Sloane Stanley GH (1957) A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 226:497–509
Skipski VP, Good JJ, Barclay M, Reggio RB (1968) Quantitative analysis of simple lipid classes by thin-layer chromatography. Biochim Biophys Acta 152:10–19
Makrides M, Neumann MA, Byard RW, Simmer K, Gibson RA (1994) Fatty acid composition of brain, retina, and erythrocytes in breast- and formula-fed infants. Am J Clin Nutr 60:189–194
Rapoport SI (2008) Arachidonic acid and the brain. J Nutr 138:2515–2520
Rabin O, Drieu K, Grange E, Chang MC, Rapoport SI, Purdon AD (1998) Effects of EGb 761 on fatty acid reincorporation during reperfusion following ischemia in the brain of the awake gerbil. Mol Chem Neuropathol 34:79–101
Coleman RA, Lewin TM, Van Horn CG, Gonzalez-Baro MR (2002) Do long-chain acyl-CoA synthetases regulate fatty acid entry into synthetic versus degradative pathways? J Nutr 132:2123–2126
Knudsen J, Neergaard TB, Gaigg B, Jensen MV, Hansen JK (2000) Role of acyl-CoA binding protein in acyl-CoA metabolism and acyl-CoA-mediated cell signaling. J Nutr 130:294S–298S
Bazan NG, Rodriguez de Turco EB (1980) Membrane lipids in the pathogenesis of brain edema: phospholipids and arachidonic acid, the earliest membrane components changed at the onset of ischemia. Adv Neurol 28:197–205
Kovalchuk Y, Miller B, Sarantis M, Attwell D (1994) Arachidonic acid depresses non-NMDA receptor currents. Brain Res 643:287–295
Surette ME, Krump E, Picard S, Borgeat P (1999) Activation of leukotriene synthesis in human neutrophils by exogenous arachidonic acid: inhibition by adenosine A(2a) receptor agonists and crucial role of autocrine activation by leukotriene B(4). Mol Pharmacol 56:1055–1062
Li RC, Row BW, Gozal E, Kheirandish L, Fan Q, Brittian KR, Guo SZ, Sachleben LR Jr, Gozal D (2003) Cyclooxygenase 2 and intermittent hypoxia-induced spatial deficits in the rat. Am J Respir Crit Care Med 168:469–475
Farkas E, Donka G, de Vos RA, Mihaly A, Bari F, Luiten PG (2004) Experimental cerebral hypoperfusion induces white matter injury and microglial activation in the rat brain. Acta Neuropathol (Berl) 108:57–64
Rosenberger TA, Villacreses NE, Hovda JT, Bosetti F, Weerasinghe G, Wine RN, Harry GJ, Rapoport SI (2004) Rat brain arachidonic acid metabolism is increased by a 6-day intracerebral ventricular infusion of bacterial lipopolysaccharide. J Neurochem 88:1168–1178
Lee H, Villacreses NE, Rapoport SI, Rosenberger TA (2004) In vivo imaging detects a transient increase in brain arachidonic acid metabolism: a potential marker of neuroinflammation. J Neurochem 91:936–945
Marcheselli VL, Bazan NG (1996) Sustained induction of prostaglandin endoperoxide synthase-2 by seizures in hippocampus. Inhibition by a platelet-activating factor antagonist. J Biol Chem 271:24794–24799
Kaufmann WE, Andreasson KI, Isakson PC, Worley PF (1997) Cyclooxygenases and the central nervous system. Prostaglandins 54:601–624
Miettinen S, Fusco FR, Yrjanheikki J, Keinanen R, Hirvonen T, Roivainen R, Narhi M, Hokfelt T, Koistinaho J (1997) Spreading depression and focal brain ischemia induce cyclooxygenase-2 in cortical neurons through N-methyl-d-aspartic acid-receptors and phospholipase A2. Proc Natl Acad Sci USA 94:6500–6505
Farooqui AA, Horrocks LA (1991) Excitatory amino acid receptors, neural membrane phospholipid metabolism and neurological disorders. Brain Res Brain Res Rev 16:171–191
Clark JD, Schievella AR, Nalefski EA, Lin LL (1995) Cytosolic phospholipase A2. J Lipid Mediat Cell Signal 12:83–117
Dennis EA (1994) Diversity of group types, regulation, and function of phospholipase A2. J Biol Chem 269:13057–13060
Bolanos JP, Almeida A (1999) Roles of nitric oxide in brain hypoxia-ischemia. Biochim Biophys Acta 1411:415–436
Rodrigo J, Fernandez AP, Serrano J, Peinado MA, Martinez A (2005) The role of free radicals in cerebral hypoxia and ischemia. Free Radic Biol Med 39:26–50
Irikura K, Morii S, Miyasaka Y, Yamada M, Tokiwa K, Yada K (1996) Impaired autoregulation in an experimental model of chronic cerebral hypoperfusion in rats. Stroke 27:1399–1404
Acknowledgments
This research was supported entirely by the Intramural Research Program of the National Institute on Aging, and by the National Institute of Environmental Health Science, NIH.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bhattacharjee, A.K., White, L., Chang, L. et al. Bilateral Common Carotid Artery Ligation Transiently Changes Brain Lipid Metabolism in Rats. Neurochem Res 37, 1490–1498 (2012). https://doi.org/10.1007/s11064-012-0740-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-012-0740-2