Abstract
During flurothyl seizures in 4-day-old rats, cortical concentration of ATP, phosphocreatine and glucose fell while lactate rose. Cortical energy use rate more than doubled, while glycolytic rate increased fivefold. Calculation of the cerebral metabolic balance during sustained seizures suggests that energy balance could be maintained in hyperglycemic animals, and would decline slowly in normoglycemia, but would be compromised by concurrent hypoglycemia, hyperthermia or hypoxia. These results suggest that the metabolic challenge imposed on the brain by this model of experimental neonatal seizures is milder than that seen at older ages, but can become critical when associated with other types of metabolic stress.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Epileptic seizures are common in premature infants and neonates, and their effect on brain metabolism and brain development has been highly controversial. In human premature or term neonates, some types of seizures are strongly associated with later cognitive deficits [18], but it is not clear whether this reflects an adverse effect of seizures on brain development, or the independent generation of seizures and cognitive loss by intercurrent illness [3, 50]. In experimental animals, repetitive seizures can impair brain growth [36, 48, 54] and cause caspase-dependent neuronal necrosis and apoptosis [14, 21, 23, 31, 37, 40, 41], but these effects are highly model-, age- and species-dependent, some seizure models may cause no cell death [1, 9, 10, 35, 43], and seizures take far longer to generate adverse effects in the developing brain than in the adult brain [18, 51]. These differences may in part reflect differences in cerebral energy metabolism. In adult animals, ATP invariably declines during seizures. This decline is rapid in convulsing rats and mice, and slower when the systemic effects of anoxia and convulsions are prevented by paralysis and oxygenation [27], but even in the absence of systemic complications, repetitive seizures compromise cerebral energy balance [2, 4, 6, 11, 17, 20, 29, 33, 34, 39] and cause widespread neuronal death [28]. Neonates have a much lower cerebral metabolic rate than adults [5, 46]. Studies in newborn dogs showed reductions of brain phosphocreatine and prolonged elevations of inorganic phosphate and lactate after seizures induced by electroshock [55] or bicuculline [32], suggesting enhanced glycolysis. In a previous study [52], we found that in 4-day-old rats, an age roughly comparable to a human premature or term neonate, brain ATP concentrations measured 1 min after the end of exposure to the convulsant flurothyl did not show the progressive depletion of energy reserves with repeated seizures during the course of status epilepticus which has been described in adults. These results, however, did not rule out a transient decline during seizures. In the present study, we determined the cortical metabolic rate and measured energy reserves in the brain of 4-day-old rats during flurothyl seizures. Results indicate that, in the neonate, flurothyl seizures elevate the rate of energy use and deplete energy reserves, but this depletion is transient unless a “second hit” such as hypoglycemia, hypoxia or hyperthermia further compromises energy supply.
Methods
Chemicals
Flurothyl was obtained from Ohio Chemical Company and other chemicals were purchased from Sigma (St. Louis, MO). Enzymes were obtained from Boehringer Mannheim Corporation (New York City), except for beef heart lactate dehydrogenase (Worthington Biochemical Corp., Freehold, New Jersey) and glycogen phosphorylase (Sigma).
Animals
Pregnant Wistar rats (CSN strain, Carwoth, New York) were housed individually and kept in a room with a 7 a.m.–7 p.m. light/dark cycle. After delivery, each litter was kept with its mother. Littermates were paired by sex and body weight (±0.5 grams) at age 4 days. One member of each pair was subjected to seizures at the age of 4 days; its companion was handled in a similar fashion but no seizures were induced. The protocols used were compliant with the NIH guidelines for protection of experimental animals at the time of the study, all experiments were conducted with the approval and in accordance with the regulations of the Institutional Animal Care and Use Committee of Sepulveda VA Medical Center.
Seizures
Four-day-old rats were held in 1 liter containers partially immersed in a water bath maintained at 36°C. Previous studies showed that under these conditions body temperature averaged 33°C, which is physiological for rat pups in the nest [7]. Flurothyl (50 μl) injected against the inner wall of the jar vaporized immediately, and the lid was closed. The oxygen content of the jar, measured with an oxygen-sensitive electrode, remained similar to that of room air throughout the experiment. Electroencephalograms were recorded in a separate population of animals. All animals exposed to flurothyl developed hyperactivity followed within 3 min by a severe tonic seizure. They were decapitated at the height of their tonic extension and the head was immediately frozen in liquid nitrogen. For determination of energy use rate, animals were decapitated at the same time, but the severed head was replaced in the immersed containers for 30 s, then frozen in liquid nitrogen. Appropriate controls were sacrificed in a similar fashion.
Chemical Methods
After freezing by immersion with vigorous agitation in liquid nitrogen, specimens were stored at −80º until they could be prepared for analysis. Cerebral cortex was dissected in a −20º room and powdered under liquid nitrogen. A weighed portion of the frozen powder (approximately 100 mg) was then layered over three volumes of frozen 3.0 M perchloric acid and homogenized in an alcohol-dry ice bath kept at −10°C. Ten volumes of cold 5 mM edetic acid were added, and the entire mixture re-homogenized and centrifuged at 5,000 g for 30 min at 0°C. The supernatant fluids were neutralized with 2 M potassium bicarbonate to pH 6.5–6.8. Precipitated potassium perchlorate was removed by centrifugation; the supernatant fluids were assayed for adenosine triphosphate (ATP), phosphocreatine, glucose, glycogen and lactate by enzymatic, fluorometric methods, in which the fluorescence of NADPH formed after addition of appropriate enzymes and NADP is measured. Glucose, ATP and phosphocreatine were measured by the methods of Lowry and Passonneau [25], lactate was determined from the formation of NADPH after addition to tissue extracts of lactate dehydrogenase and NADP by the method of Vannucci and Duffy [45], and glycogen by the method of Passonneau et al. [33].
Energy use Rates
Since under decapitation conditions there is no exchange with the environment, the initial rate of disappearance of compounds in which energy is stored is a measure of utilization of energy in steady state, as suggested by Lowry et al. [24] and Gatfield et al. [16]. Assuming that during the next 30 s, energy is utilized at the same rate as immediately prior to decapitation, the difference between high energy bonds before and after decapitation is a measure of the steady rate of energy use immediately prior to decapitation.
Alternatively, the energy use rate can be calculated using the rate of lactate formation (since the severed head is a closed system in which no 02 is available) instead of the rate of utilization of glucose and glycogen. Δ ~P = 2 Δ ATP + Δ phosphocreatine + Δ lactate.
Energy use rates were calculated by both methods. Values displayed in Fig. 1 represent an average of the values obtained by both methods. Energy produced from glycolysis was calculated as the sum of high-energy phosphate bonds produced from glucose and glycogen: Δ ~Pg = Δ glucose + 2.9 Δ glycogen [42]. Energy reserves were calculated as the sums of high-energy phosphate bonds that could be produced by metabolism of all glycogen, glucose, ATP and phosphocreatine in the absence of oxygen: ~P reserves = 2 [ATP] + [phosphocreatine] + 2 [glucose] + 2.9 [glycogen].
Results
Following injection of flurothyl in the jar, 4-day-old rats became progressively hyperactive. Recordings in a parallel population showed isolated epileptifrom spikes during that period of hyperactivity. This was followed by a tonic seizure, electrographically characterized by high voltage polyspikes. Brains frozen at the time of tonic extension showed a 15% fall in cortical ATP, a 44% decline in phosphocreatine (Table 1), and an 87% increase in lactate concentration (Fig. 1). The cortical metabolic rate was calculated by two different methods. Based on the decline of the energy reserves in the decapitated head, it went from 2.6 mmoles of high energy phosphate bonds per kg per min to 7.40 mmoles/kg/min, a 185% increase. The same rates were also calculated based on the increase in lactate in the decapitated head, yielding rates of 3.16 mmoles of high energy phosphate equivalents per kg per min in controls and 5.58 mmoles/kg/min in seizing animals, a 77% increase. Based on the average of those two values, the calculated metabolic rate at least doubled during a single, brief seizure in 4-day-old rats. ATP concentration fell significantly and phosphocreatine concentration fell by nearly half, but energy reserves declined only slightly, from 17.43 mmoles ~P/kg to 16.84 mmoles/kg, reflecting both the short seizure duration and the large glycogen reserves which showed no significant mobilization. This moderate decline in energy reserves and increase in lactate occurred at the time of initial tonic extension, which in the average animal takes place within 10–15 s of the onset of generalized polyspike activity.
Discussion
These data show that in the brain of the neonate as in the adult, generalized convulsive seizures result, within a few seconds, in an increase in metabolic rate, decline in energy reserves, and increase in lactate. The increase in energy use outstripped the tissue’s ability to reconstitute its energy reserves or to use its glycogen, suggesting that the neonate, like the adult, uses more energy than it can generate during the tonic phase of seizures. Since 4-day-old rats during flurothyl seizures are hypoxemic [49], we do not know if those results would also apply to paralyzed and 02-ventilated animals. Status epilepticus is immature marmosets depletes energy reserves even when seizures do not produce hypoxemia or hypotension [15], but flurothyl status epilepticus in newborn dogs does not [56]. The fall in ATP probably accounts are least in part for the inhibition of protein synthesis observed during seizures in those neonates [48] through a GDP cascade effect mediated by the enzyme nucleotide kinase [12].
Our previous investigations [52] showed no decline in post-ictal ATP during the course of status epilepticus in 4-day-old rats, but the animals were frozen during the post-ictal period, 1 min after removal of the convulsant. ATP concentrations were not measured during the seizures in that study. Those results do indicate, however, that ATP depletion in newborn rats is rapidly reversible when seizures stop.
The large increase in cortical metabolic rate suggests that neonatal seizures involved neocortex in spite of its immaturity. In fact, cortical glycolytic rates increased fivefold in our animals. The discrepancy between cerebral metabolic rates calculated from the fall in glucose and glycogen, and those calculated from the rise in lactate, was expected. With short decapitation times such as the 30 s used here, the minimal oxygen stores of the severed head should be sufficient to significantly reduce initial lactate formation. As a result, the lactate method may slightly underestimate the true cerebral metabolic rate [46]. Both the magnitude of the changes and the metabolic rate might also have been underestimated, because of the difficulty of maintaining the temperature of the small severed head which has a high surface to volume ratio. However, any underestimation would be slight because of the short times involved.
In decapitated animals, glucose declines rapidly, but glycogen only shows small changes. Neonates lack the ability to generate large amount of cyclic AMP to activate phosphorylase [53], and their brain phosphorylase concentrations are low. As a result, glycogen mobilization is quite slow and limits the tissue’s ability to maintain critical metabolite levels, in spite of the presence of large glycogen reserves [44, 47].
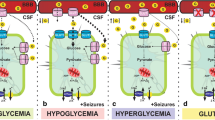
Based on the very large increases in metabolic rate measured, we can use a model of brain energy metabolism to calculate whether the immature rat cortex can generate enough high-energy phosphate bonds to maintain metabolic balance during prolonged seizures. The calculated transport capacity of the rat pup’s blood–brain–barrier is 0.089, 0.168 and 0.23 mmoles/kg/min of glucose, at plasma glucose concentrations of 2 mM, 5 mM and 20 mM respectively. In a fully oxygenated brain, 1 mol of glucose generates 38 mol of ATP. At blood glucose concentrations of 20 mM, the 0.23 mmoles glucose/kg/min transported into the brain can generate 7.54 mmoles ~P/kg/min (assuming that 15% of the glucose is directed to lactate formation). This is sufficient to maintain the energy use rate measured at the onset of seizures, and to preserve energy balance indefinitely. However, if blood glucose is 5 mM, there is a putative deficit of 1.9 mmoles ~P/kg/min. Even assuming that ketone bodies can produce one third of the resting metabolic rate (0.9 mmoles ~P/kg/min) and that glycogen can be mobilized at a rate of 0.37 mmoles ~P/kg/min [47], there remains a deficit of 0.6 mmole ~P/kg/min, and energy reserves would slowly decline if the metabolic rate is maintained. At a blood glucose concentration of 2 mM, total exhaustion of energy reserves would occur in 48 min if glycogen can be mobilized, and in 12 min if it cannot. In fact, our studies have shown that in newborn rats, rabbits and marmosets, seizures deplete brain glucose within minutes, presumably as a result of the massively increased glycolytic rate [13]. Thus the energy balance of the neonatal brain during seizures is quite precarious, and blood glucose concentration is a major determinant of the timing of energy failure. The hyperglycemia which results from the release of catecholamines by seizures [8] may well have a protective role for seizing neonates.
If oxygen is not available, 1 mol of glucose only generates 2 mol of ATP, ketone bodies cannot be utilized, and rates of ~P generation would be 0.336 and 0.37 mmoles/kg/min from glucose transport into brain and from glycolysis, respectively. During a seizure, the brain of a normoglycemic neonate would run out of ATP in 2.51 min. Profound ATP depletion during hypoxic seizures has been observed [19]. Since cerebral metabolic rate increases 5.3% per degree [22], hyperthermia to 39°C would be expected to raise energy use rate during seizures in our animals to 10.09 mmole ~P/kg/min. The brain would take 82 min to completely run out of ATP, but the level of depletion which is critical for neuronal injury would probably be reached in a much shorter time [26, 38].
It should be stressed that while there is no true human equivalent of the developmental stage of a P4 rat, the age selected for this study most closely approximates the developmental stage of a late-term premature infant, since rats at birth are slightly more immature than humans, and both species are predominantly post-natal brain developers. While the results of this study are highly relevant to the human neonatal period, with its slow cerebral metabolic rate and limited synaptic connections, they should not be extended to later periods of brain development, when the still immature brain in both species has a higher metabolic rate and a higher number of synaptic connections that the adult [5, 46]. We must also point out that energy use rates vary regionally and with the seizure model, so that the conclusions of this study do not necessarily apply to other seizure types and other brain regions.
In conclusion, flurothyl seizures in P4 rat pups raise energy use and glycolytic rates to a point where recovery of energy reserves can take place after each seizure in normothermic, normoglycemic, normoxic pups, but might result in critical energy depletion when concurrent hyperthermia, hypoglycemia or hypoxia occur.
References
Albala BJ, Moshé SL, Okada R (1984) Kainic-acid-induced seizures: a developmental study. Brain Res 315(1):139–148
Bain JA, Pollock GH (1949) Normal and seizures levels of lactate pyruvate and acid-double phosphates in the cerebellum and cerebrum. Proc Soc Exp Biol Med 71:495–497
Camfield PR (1997) Recurrent seizures in the developing brain are not harmful. Epilepsia 38(6):735–737
Chapman AG, Meldum BS, Siesjo BK (1977) Cerebral metabolite changes during prolonged epileptic seizures in rats. J Neurochem 28:1025–1035
Chugani HT, Phelps ME (1986) Maturational changes in cerebral function in infants determined by 18FDG positron emission tomography. Science 231(4740):840–843
Collins RC, Posner JB, Plum F (1970) Cerebral energy metabolism during electroshock seizures in mice. Am J Physiol 218:943–950
Conklin P, Heggeness FW (1971) Maturation of temperature homeostasis in the rat. Am J Physiol 220:333–336
Devinsky O, Emoto S, Goldstein DS, Stull R, Porter RJ, Theodore WH, Nadi NS (1992) Cerebrospinal fluid and serum levels of dopa, catechols, and monoamine metabolites in patients with epilepsy. Epilepsia 33(2):263–270
Dubé CM, Brewster AL, Richichi C, Zha Q, Baram TZ (2007) Fever, febrile seizures and epilepsy. Trends Neurosci 30(10):490–496
Dubé CM, Ravizza T, Hamamura M, Zha Q, Keebaugh A, Fok K, Andres AL, Nalcioglu O, Obenaus A, Vezzani A, Baram TZ (2010) Epileptogenesis provoked by prolonged experimental febrile seizures: mechanisms and biomarkers. J Neurosci 30(22):7484–7494
Duffy TE, Howse DC, Plum F (1975) Cerebral energy metabolism during experimental status epilepticus. J Neurochem 24:925–934
Dwyer BE, Wasterlain CG (1980) Regulation of the first step of the initiation of brain protein synthesis by guanosine diphoophate. J Neurochem 34:1639–1647
Dwyer BE, Wasterlain CG (1986) Neonatal seizures in monkeys and rabbits: brain glucose depletion in the face of mormoglycemia, prevention by glucose loads. Pediatr Res 19:992–997
Ekstrand JJ, Pouliot WA, Scheerlinck PH, Trandafir CC, Dudek FE (2008) Lithium pilocarpine-induced status epilepticus in the immature rat results in widespread neuronal injury in the ventral hippocampus, thalamus, and amygdala. Epilepsia 49:464
Fujikawa DG, Dwyer BE, Lake RR, Wasterlain CG (1986) Local cerebral glucose utilization during status epilepticus in newborn primates. Am J Physiol 256(Cell Physiology 25):C1160–C1167
Gatfield PD, Lowry OH, Schulz DW (1966) Regional energy reserves in mouse brain and changes with ischemia and anesthesia. J Neurochem 13:185–195
Guardijan ES (1947) Cerebral metabolism in metrazol convulsions in the dog. Res Publ Assoc Nerv Mental Dis 26:184–204
Holmes GL (2009) The long-term effects of neonatal seizures. Clin Perinatol 36(4):901–914
Jensen F, Tsuji M, Offutt M, Firkusny I, Holtzman D (1993) Profound, reversible energy loss in the hypoxic immature rat brain. Brain Res Dev Brain Res 73(1):99–105
King LJ, Lowry OH, Passonneau JV, Venson V (1967) Effects of convulsants on energy reserves in the cerebral cortex. J Neurochem 14(6):599–611
Kubova H, Druga R, Lukasiuk K, Suchomelova L, Haugvicova R, Jirmanova I, Pitkanen A (2001) Status epilepticus causes necrotic damage in the mediodorsal nucleus of the thalamus in immature rats. J Neurosci 21:3593–3599
Laptook AR, Corbett RJT, Sterett R, Burns DK, Garcia D, Tollefsbol G (1997) Modest hypothermia provides partial neuroprotection when used for immediate resuscitation after brain ischemia. Pediatr Res 42:17–23
Lopez-Meraz ML, Wasterlain CG, Rocha LL, Allen S, Niquet J (2010) Vulnerability of postnatal hippocampal neurons to seizures varies regionally with their maturational stage. Neurobiol Dis 37(2):394–402
Lowry OH, Passonneau JV, Hasselberger FY (1964) Effect of glycolytic pathway in brain. J Biol Chem 239:18–30
Lowry OH, Passonneau JV (1972) A flexible system of enzymatic analysis. Academic Press, New York
Lundgren J, Smith ML, Blennow G, Siesjo BK (1994) Hyperthermia aggravates and hypothermia ameliorates epileptic brain damage, Exp. Brain Res 99(1994):43–55
Meldrum BS, Brierley JB (1973) Prolonged epileptic seizures in primates: ischemic cell changes and its relation to ictal physiological events. Arch Neurol 28:10–17
Meldrum BS, Vigouroux RA, Brierley JB (1973) Systemic factors and epileptic brain damage. Prolonged seizures in paralyzed, artificially ventilated baboons. Arch Neurol 29(2):82–87
Meric P, Barrere B, Peres M, Gillet B, Berenger G, Beloeil JC, Seylaz J (1994) Effects of kainate-induced seizures on cerebral metabolism: a combined 1H and 31P NMR study in rat. Brain Res 638(1–2):53–60
Niquet J, Auvin S, Archie M, Seo DW, Allen S, Sankar R, Wasterlain CG (2007) Status epilepticus triggers caspase-3 activation and necrosis in the immature rat brain. Epilepsia 48(6):1203–1206
Passonneau JV, Gatfield PD, Schulz DW (1967) An enzymic method for measurement of glycogen. Anal Biochem 19:315–326
Petroff OA, Prichard JW, Ogino T, Avison M, Alger JR, Shulman RG (1986) Combined 1H and 31P nuclear magnetic resonance spectroscopic studies of bicuculline-induced seizures in vivo. Ann Neurol 20(2):185–193
Plum G et al (1968) Cerebral metabolic and circulatory responses to induced convulsion in animals. Arch Neurol 18:1–13
Posner JB et al (1969) Cerebral metabolism during electrically induced seizures in man. Arch Neurol 20:388–395
Raol YS, Budreck EC, Brooks-Kayal AR (2003) Epilepsy after early-life seizures can be independent of hippocampal injury. Ann Neurol 53:503–511
Ribak CE, Baram TZ (1996) Selective death of hippocampal CA3 pyramidal cells with mossy fiber afferents after CRH-induced status epilepticus in infant rats. Brain Res Dev Brain Res 91(2):245–251
Sankar R, Shin DH, Liu H, Mazarati A, De Vasconcelos AP, Wasterlain CG (1998) Patterns of Status Epilepticus-induced neuronal injury during development and long-term consequences. J Neurosci 18:8382–8393
Toth Z, Yan XX, Haftoglou S, Ribak CE, Baram TZ (1998) Seizure-induced neuronal injury: vulnerability to febrile seizures in an immature rat model. J Neurosci 18(11):4285–4294
Stone WE (1945) Chemical changes in the cerebral cortex associated with convulsive activity. J Neurophysiol 8:233–240
Thompson KW, Wasterlain CG (1997) Lithium-pilocarpine status epilepticus in the immature rabbit. Brain Res 100:1–4
Thompson K, Holm AM, Schousboe A, Popper P, Micevych P, Wasterlain C (1998) Hippocampal stimulation produces neuronal death in the immature brain. Neuroscience 82:337–348
Thurston JH, Mcdougall DB Jr (1969) Effect of ischemia on metabolism of the brain in newborn mouse. Am J Physiol 216:348–352
Tremblay E, Nitecka L, Berger ML, Ben-Ari Y (1984) Maturation of kainic acid seizure-brain damage syndrome in the rat. I. Clinical, electrographic and metabolic observations. Neuroscience 4:1051–1072
Vannucci RC (1981) Metabolic and pathological consequences of experimental febrile seizures and status epilepticus. In: Nelson K, Ellenberger JH (eds) Febrile seizures. Raven Press, New York, pp 43–57
Vannucci RC, Duffy TE (1974) The influence of birth on carbohydrate and energy metabolism in rat brain. Am J Physiol 226:933–940
Vannucci RC, Duffy TE (1976) Carbohydrate metabolism in fetal and neonatal rat brain during anoxia and recovery. Am J Physiol 230:1269–1275
Vannucci RC, Vannucci SY (1980) Glycogen metabolism in neonatal rat brain during anoxia and recovery. J Neurochem 34:100–1105
Wasterlain CG (1976) Effect of neonatal status epilepticus on rat brain development. Neurology 26:975–986
Wasterlain CG (1979) Does anoxemia play a role in the effects of neonatal seizures on brain growth? An experimental study in the rat. Eur Neurol 18:222–229
Wasterlain CG (1997) Recurrent seizures can damage the developing brain. Epilepsia 38:728–734
Wasterlain CG, Niquet J, Liu H, Sankar R, Mazarati AM, Suchomelova L, Katsumori H, Shirasaka Y (2002) Seizure-induced neuronal death in the immature brain. In: Sutula T, Pitkanen A (eds) Do seizures damage the brain. Prog Brain Res 135:335–353
Wasterlain CG, Duffy TE (1976) Status epilepticus in immature rats. Arch Neurol 33:821–827
Wasterlain CG, Dwyer BE (1983) Brain metabolism during prolonged seizures in neonates. In: Delgado-Escueta AV, Wasterlain CG, Treiman D, Porter RJ (eds) Status epilepticus: mechanism of brain damage and treatment. Raven Press, New York, pp 241–260
Wasterlain CG, Plum F (1973) Vulnerability of developing rat brain to electroconvulsive seizures. Arch Neurol 29:38–45
Young RS, Cowan BE, Petroff OA, Novotny E, Dunham SL, Briggs RW (1987) In vivo 31P and in vitro 1H nuclear magnetic resonance study of hypoglycemia during neonatal seizure. Ann Neurol 22(5):622–628
Young RSK, Petroff OAC, Chen B, Gore JC, Aquila WJ (1991) Brain Energy state and lactate metabolism during status epilepticus in the neonatal dog: in vivo 31P and 1H nuclear magnet resonance study. Pediatr Res 29:191–195
Acknowledgments
Supported by VHA Research Service and by research grant RO1-NS13515 from NINDS. A large part of the credit for this work belongs to Dr. Thomas E. Duffy, who unfortunately died before its completion. We are indebted to Barbara Blackburn for preparation of the manuscript, and to Roger Baldwin for superb technical help. I (CGW) am particularly indebted to Dr Abel Lajtha for his support during my early days as a neurochemist. He was both a mentor and an inspiration, and was always available and generous with fresh ideas, new techniques and very wise advice. Abel and Claude Baxter welcomed me into the Society for Neurochemistry, which was a young and vibrant meeting place for all kinds of scientists. Abel was a strong leader, but his style was so kind and unassuming that I always felt like he was helping me to find my own way. There are only a few people whose influence on the world has been exclusively positive, and he is one of those elite few. I hope that he will continue to provide wisdom and inspiration to present and future neurochemists.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Additional information
Special Issue: In Honor of Dr. Abel Lajtha.
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Wasterlain, C.G., Thompson, K.W., Suchomelova, L. et al. Brain Energy Metabolism During Experimental Neonatal Seizures. Neurochem Res 35, 2193–2198 (2010). https://doi.org/10.1007/s11064-010-0339-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-010-0339-4