Abstract
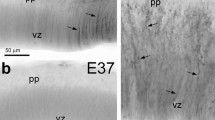
Astrocytes have been considered to be transformed from radial glial cells that appear at early stage of development and play a scaffold-role for neuronal cell migration. Recent studies indicate that neuroepithelial cells in the spinal cord also give rise to astrocytes. However, the mode of astroglial generation and migration in the ventricular neuroepithelium remains poorly understood. In this study, we have utilized immunohistochemical and retroviral lineage tracing methods to characterize the developmental profiles of astrocytes in the chick optic tectum, which develops from both the neural tube and invasion of optic tract. Chick vimentin and glial fibrillary acidic protein (GFAP) were found as single bands at molecular weights consistent with those reported for mammalian species. Differential developmental trends were observed for both proteins with relative vimentin levels decreasing and GFAP levels increasing with embryonic age. We observed two streams of tectal GFAP-labeled astrocytes originated from the tectal ventricle (intrinsic origin) and the optic tract (extrinsic origin). The extrinsic astrocytes arose from the ventral neuroepithelium of the third ventricle, dispersed bilaterally to the optic tract, and subsequently to the outer layer of optic tectum, indicating migration of astrocytes along retinal ganglion cell axons. On the other hand, the intrinsic astrocytes from the tectal ventricular neuroepithelium appeared first in the ventral part of the optic tectum, and then in the lateral and dorsal tectum. The intrinsic tectal astrocytes closely associated with fascicles of vimentin-labeled radial glial cells, indicating a presumptive radial migration of astrocytes. These results demonstrated that the optic tectum contains heterogeneous populations of astrocytes developed from the different origins and routes of migration.









Similar content being viewed by others
References
Warf BC, Fok-Seang J, Miller RH (1991) Evidence for the ventral origin of oligodendrocyte precursors in the rat spinal cord. J Neurosci 11:2477–2488
Pringle NP, Richardson WD (1993) A singularity of PDGF alpha-receptor expression in the dorsoventral axis of the neuronal tube may define the origin of the oligodendrocytes lineage. Development 117:525–533
Yu W-P, Collarini EJ, Pringle NP et al (1994) Embryonic expression of myelin genes: evidence for a focal source of oligodendrocytes precursors in the ventricular zone of the neural tube. Neuron 12:1353–1362
Timsit S, Martinez S, Allinquant B et al (1995) Oligodendrocytes originate in a restricted zone of the embryonic ventral neural tube defined by DM-20 mRNA expression. J Neurosci 15:1012–1024
Ono K, Yasui Y, Rutishauser U et al (1997) Focal ventricular origin and migration of oligodendrocyte precursors into the chick optic nerve. Neuron 19:283–292
Galileo DS (2003) Spatiotemporal gradient of oligodendrocyte differentiation in chick optic tectum requires brain integrity and cell-cell interactions. Glia 41:25–37
Bignami A, Dahl D (1974) Astrocyte-specific protein and radial glia in the cerebral cortex of newborn rat. Nature 252:55–56
Benjelloun-Touimi S, Jacque CM, Derer P et al (1985) Evidence that mouse astrocytes may be derived from the radial glia. An immunohistochemical study of the cerebellum in the normal and reeler mouse. J Neuroimmunol 9:87–97
Voigt T (1989) Development of glial cells in the cerebral wall of ferrets: direct tracing of their transformation from radial glia into astrocytes. J Comp Neurol 289:74–88
Culican SM, Baumrind NL, Yamamoto M et al (1990) Cortical radial glia: identification in tissue culture and evidence for their transformation to astrocytes. J Neurosci 10:684–692
Dahl D (1981) The vimentin-GFA transition in rat neuroglia cytoskeleton occurs at the time of myelination. J Neurosci Res 6:741–748
Pixley SR, De Vellis J (1984) Transition between immature radial glia and the mature astrocytes studies with monoclonal antibody to vimentin. Brain Res Dev Brain Res 15:201–209
McDermott KW, Lantos PL (1989) The distribution of glial fibrillary acidic protein and vimentin in postnatal marmorset (Callithrix jacculus) bain. Brain Res Dev Brain Res 45:169–177
Dahl D, Bignami A (1973) Immunohistochemical and immunofluorescence studies of the glial fibrillary acidic protein in vertebrates. Brain Res 61:279–283
Onteniente B, Kimura H, Maeda T (1983) Comparative study of the glial fibrillary acidic protein in vertebrates by PAP immunohistochemistry. J Comp Neurol 215:427–436
Dahl D, Crosby CJ, Sethi A et al (1985) Glial fibrillary acidic (GFA) protein in vertebrates: immunofluorescence and immunoblotting study with monoclonal and polyclonal antibodies. J Comp Neurol 239:75–88
Alvarez-Buylla A, Buskirk DR, Nottebohm F (1987) Monoclonal antibody reveals radial glia in adult avian brain. J Comp Neurol 264:159–170
Kálmán M, Székely AD, Csillag A (1998) Distribution of glial fibrillary acidic protein and vimentin-immunopositive elements in the developing chicken brain from hatch to adulthood. Anat Embryol 198:213–235
Pringle NP, Yu W-P, Howell M et al (2003) Fgfr3 expression by astrocytes and their precursors: evidence that astrocytes and oligodendrocytes originate in distinct neuroepithelial domains. Development 130:93–102
Ivanova A, Agochiya M, Amoyel M et al (2004) Receptor tyrosine phosphatase zeta/beta in astrocyte progenitors in the developing chick spinal cord. Gene Expr Patterns 4:161–166
Galileo DS, Gray GE, Owens GC et al (1990) Neurons and glia arise from a common progenitor in chicken optic tectum: Demonstration with two retroviruses and cell type-specific antibodies. Proc Natl Acad Sci USA 87:458–462
Gray GE, Sanes JR (1992) Lineage of radial glia in the chicken optic tectum. Development 114:271–283
Fu H, Cai J, Rutledge M et al (2003) Oligodendrocytes can be generated from the local ventricular and subventricular zones of embryonic chicken midbrain. Brain Res Dev Brain Res 143:161–165
Kim DW, Park SW, Jeon GS et al (2006) The multiple dorsoventral origins and migratory pathway of tectal oligodendrocytes in the developing chick. Brain Res 1076:16–24
Hamburger V, Hamlton HL (1951) A series of normal changes in the development of the chick embryo. J Morphol 88:49–92
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Cho SS, Hyndman AG (1991) The ontogeny of transferrin receptors in the embryonic chick retina: an immunohistochemical study. Brain Res 549:375–387
Cepko CL, Ryder E, Austin C et al (1998) Lineage analysis using retroviral vectors. Methods Enzymol 14:393–406
LaVail JH, Cowan WM (1971) The development of the chick optic tectum. I. Normal morphology and cytoarchitectonic development. Brain Res 28:391–419
Mey J, Thanos S (2000) Development of the visual system of the chick. I. Cell differentiation and histogenesis. Brain Res Brain Res Rev 32:343–379
Kakita A, Zerlin M, Takahashi H et al (2003) Some glial progenitors in the neonatal subventricular zone migrate through the corpus callosum to the contralateral cerebral hemisphere. J Comp Neurol 458:381–388
Ling TL, Stone J (1988) The development of astrocytes in the cat retina: evidence of migration from the optic nerve. Brain Res Dev Brain Res 44:73–85
Watanabe T, Raff MC (1988) Retinal astrocytes are immigrants from the optic nerve. Nature 332:834–837
Choi KW, Benzer S (1994) Migration of glia along photoreceptor axons in the developing Drosophila eye. Neuron 12:423–431
Steiner B, Kronenberg G, Jessberger S et al (2004) Differential regulation of gliogenesis in the context of adult hippocampal neurogenesis in mice. Glia 46:41–52
Malatesta P, Hartfuss E, Götz M (2000) Isolation of radial glial cells by fluorescent-activated cell sorting reveals a neuronal lineage. Development 127:5253–5263
Chanas-Sacre G, Rogister B, Moonen G et al (2000) Radial glia phenotype: origin, regulation and transdifferentiation. J Neurosci Res 61:357–363
Davies JE, Miller RH (2001) Local sonic hedgehog signaling regulates oligodendrocytes precursor appearance in multiple ventricular zone domains in the chick metencephalon. Dev Biol 233:513–525
Zerlin M, Levison SW, Goldman JE (1995) Early patterns of migration, morphogenesis, and intermediate filament expression of subventricular zone cells in the postnatal rat forebrain. J Neurosci 15:7238–7249
Diers-Fenger M, Kirchhoff F, Kettenmann H et al (2001) AN2/NG2 protein-expressing glial progenitor cells in the murine CNS: isolation, differentiation, and association with radial glia. Glia 34:213–228
Acknowledgments
This work was supported by grant No. R01-2003-000-10478-0 from the Basic Research Program of the Korea Science & Engineering Foundation and by research grant of the Chungbuk National University in 2006. This study was also supported partially by the second stage Brain Korea 21 Project in 2007 and a grant (M103KV010018 04K2201 01850) from Brain Research Center of the 21st Century Frontier Research Program funded by the Ministry of Science and Technology, the Republic of Korea.
Author information
Authors and Affiliations
Corresponding author
Additional information
J.H. Seo and J.H. Chang contributed equally to this work.
Rights and permissions
About this article
Cite this article
Seo, J.H., Chang, J.H., Song, S.H. et al. Spatiotemporal Gradient of Astrocyte Development in the Chick Optic Tectum: Evidence for Multiple Origins and Migratory Paths of Astrocytes. Neurochem Res 33, 1346–1355 (2008). https://doi.org/10.1007/s11064-008-9590-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-008-9590-3