Abstract
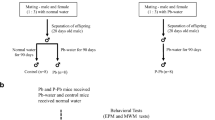
To determine the effect of prenatal lead exposure on brain monoaminergic systems, pregnant rats were given tap water containing 250 ppm lead acetate, for the duration of pregnancy, while tap water without lead (Pb2+) was substituted at birth. Control rats were derived from dams that consumed tap water during pregnancy, and had no exposure to lead afterwards. At 12 weeks after birth, Pb2+ content of brain cortex was increased 3- to 4-fold (P < 0.05). At this time the endogenous striatal levels of 3,4-dihydroxyphenylacetic acid (DOPAC) and homovanillic acid were 19% lower in Pb2+ exposed rats (P < 0.05), while there was no change in the striatal level of dopamine (DA), noradrenaline, 3,4-dihydroxyphenylglycol, serotonin (5-HT) and 5-hydroxyindoleacetic acid (HPLC/ED). Also there was no change in these monoamines and metabolites in the prefrontal cortex of Pb2+ exposed rats. However, turnover of 5-HT in prefrontal cortex, as indicated by 5-hydroxytryptophan accumulation 30 min after acute treatment with the decarboxylase inhibitor NSD-1015 (100 mg/kg IP), was lower in the Pb2+ exposed rats. In the striatum AMPH-induced (1 mg/kg IP) turnover of DA, evidenced as L-DOPA accumulation after NSD-1015, was increased to a lesser extent in the Pb2+ exposed rats (P < 0.05). The nitric oxide synthase inhibitor 7-nitroindazole (10 mg/kg IP) attenuated the latter effect, indicating that neuronal NO mediates this AMPH effect, at least in part. Moreover, DA D2 receptor sensitivity developed in Pb2+ exposed rats, as evidenced by enhanced quinpirole-induced yawning activity and enhanced quinpirole-induced locomotor activity (each, P < 0.05). These findings indicate that ontogenetic exposure to lead can have consequences on monoaminergic neuronal function at an adult stage of life, generally promoting accentuated behavioral effects of direct and indirect monoaminergic agonists, and related to increased dopamine turnover in basal ganglia.





Similar content being viewed by others
References
Bradbury MW, Deane R (1993) Permeability of the blood-brain barrier to lead. Neurotoxicology 14:131–136
Guilarte TR, McGlothan JL (1998) Hippocampal NMDA receptor mRNA undergoes subunit specific changes during developmental lead exposure. Brain Res 790:98–107
Leret ML, Garcia-Uceda F, Antonio MT (2002) Effects of maternal lead administration on monoaminergic, GABAergic and glutamatergic systems. Brain Res Bull 58:469–473
Devi CB, Reddy GH, Prasanthi RP, Chetty CS, Reddy GR (2005) Developmental lead exposure alters mitochondrial monoamine oxidase and synaptosomal catecholamine levels in rat brain. Int J Dev Neurosci 23:375–381
Antonio MT, Leret ML (2000) Study of the neurochemical alterations produced in discrete brain areas by perinatal low-level lead exposure. Life Sci 67:635–642
Devoto P, Flore G, Ibba A, Fratta W,and Pani L (2001) Lead intoxication during intrauterine life and lactation but not during adulthood reduces nucleus accumbens dopamine release as studied by brain microdialysis. Toxicol Lett 121:199–206
Ma T, Chen HH, Ho IK (1999) Effects of chronic lead (Pb) exposure on neurobehavioral function and dopaminergic neurotransmitter receptors in rats. Toxicol Lett 105:111–121
Gedeon Y, Ramesh GT, Wellman PJ, Jadhav AL (2001) Changes in mesocorticolimbic dopamine and D1/D2 receptor levels after low level lead exposure: a time course study. Toxicol Lett 123:217–226
Brus R, Szkilnik R, Nowak P, Konecki J, Głowacka M, Kasperska A, Oświęcimska J, Sawczuk K, Shani J (1997) Prenatal exposure of rats to lead, induce changes in the reactivity of the central dopaminergic, serotoninergic and muscarinic receptors, but not in glucose uptake in their offspring. Pharmacol Rev Comm 9:299–310
Brus R, Szkilnik R, Nowak P, Oświęcimska J, Kasperska A, Sawczuk K, Słota P, Kwieciński A, Kubański N, Shani J (1999) Effect of lead and ethanol, consumed by pregnant rats, on behavior of their grown offsprings. Pharmacol Rev Comm 10:175–186
Szkilnik R, Nowak P, Winiarska H, Durczok A, Małecki S, Rycerska A, Brus R, Shani J (2001) Effect of zinc on the reactivity of the central dopamine receptors in rats prenatally exposed to lead. Pharmacol Rev Comm 11:319–328
Durczok A, Szkilnik R, Brus R, Nowak P, Labus Ł, Konecki J, Drabek K, Kuballa G, Rycerski W, Mengel K (2002) Effect of organic mercury exposure during early stage of ontogenic development on the central dopaminergic system in adult rats. Pol J Environ Stud 11:307–314
Kiszka W, Szkilnik R, Brus R, Nowak P, Konecki J, Durczok A, Mengel K, Shani J (2002) Prenatal exposure of rats to mercury induces changes in central dopaminergic activity and in glucose uptake by their offspring. Pharmacol Rev Comm 12:77–90
Nowak P, Brus R, Szkilnik R, Labus L, Winiarska H, Shani J (2002b) Exposure of pregnant rats to ethanol and cadmium modulates levels of biogenic amines in brains of their adult offspring. Pharmacol Rev Comm 12:229–239
Nowak P, Dąbrowska J, Bortel A, Biedka I, Kostrzewa RM, Brus R (2006) Prenatal cadmium and ethanol increase amphetamine-evoked dopamine release in rat striatum. Neurotoxicol Teratol 28:563–572
Nation JR, Miller DK, Bratton GR (2000) Developmental lead exposure alters the stimulatory properties of cocaine at PND 30 and PND 90 in the rat. Neuropsychopharmacology 23:444–454
Blazka ME, Harry GJ, Luster MI (1994) Effect of lead acetate on nitrite production by murine brain endothelial cell cultures. Toxicol Appl Pharmacol 126:191–194
Tian L, Lawrence DA (1995) Lead inhibits nitric oxide production in vitro by murine splenic macrophages. Toxicol Appl Pharmacol 132:156–163
Vaziri ND, Ding Y, Ni Z (2001) Compensatory up-regulation of nitric-oxide synthase isoforms in lead-induced hypertension; reversal by a superoxide dismutase-mimetic drug. J Pharmacol Exp Ther 298:679–685
Garber MM, Heiman AS (2002) The in vitro effects of Pb acetate on NO production by C6 glial cells. Toxicol In Vitro 16:499–508
Garcia-Arenas G, Claudio L, Perez-Severiano F, Rios C (1999) Lead acetate exposure inhibits nitric oxide synthase activity in capillary and synaptosomal fractions of mouse brain. Toxicol Sci 50:244–248
Trabace L, Kendrick KM (2000) Nitric oxide can differentially modulate striatal neurotransmitter concentrations via soluble guanylate cyclase and peroxynitrite formation. J Neurochem 75:1664–1674
Marchetti C (2003) Molecular targets of lead in brain neurotoxicity. Neurotox Res 5:221–236
Brus R, Kostrzewa RM, Perry KW, Fuller RW (1994) Supersensitization of the oral response to SKF 38393 in neonatal 6-hydroxydopamine-lesioned rats is eliminated by neonatal 5,7-dihydroxytryptamine treatment. J Pharmacol Exp Ther 268:231–237
Kostrzewa RM, Brus R, Rykaczewska M, Plech A (1993) Low dose quinpirole ontogenetically sensitizes to quinpirole-induced yawning in rats. Pharmacol Biochem Behav 44:487–489
Whiteside P (1976) Atomic absorption. Pye Unicam Ltd., Cambridge, England
Nowak P, Brus R, Kostrzewa RM (2001) Amphetamine-induced enhancement of neostriatal in vivo microdialysate dopamine content in rats, quinpirole-primed as neonates. Pol J Pharmacol 53:319–329
Wagner J, Vitali P, Palfreyman MG, Zraika M, Hout S (1982) Simultaneous determination of 3,4-dihydroxyphenylalanine, 5-hydroxytryptophan, dopamine, 4-hydroxy-3-metoxyphenylalanine, norepinephrine, 3,4-dihydroxyphenylacetic acid, homovanillic acid, serotonin, and 5-hydroxyindoleacetic acid in rat cerebrospinal fluid and brain by high-performance liquid chromatography with electrochemical detection. J Neurochem 38:1241–1254
Carlsson A, Davis JN, Kher W, Lindqvist M, Atack CV (1972) Simultaneous measurement of tyrosine and tryptophan hydroxylase activities in brain in vivo using an inhibitor of the aromatic amino acid decarboxylase. Naunyn-Schmiedeberg’s Arch Pharmacol 275:153–168
Guilarte TR, McGlothan JL (2003) Selective decrease in NR1 subunit splice variant mRNA in the hippocampus of Pb2+-exposed rats: implications for synaptic targeting and cell surface expression of NMDAR complexes. Brain Res Mol Brain Res 113:37–43
Nowak P, Brus R, Oswiecimska J, Sokola A, Kostrzewa RM (2002a) 7-Nitroindazole enhances amphetamine-evoked dopamine release in rat striatum. an in vivo microdialysis and voltammetric study. J Physiol Pharmacol 53:251–263
Cory-Slechta DA, Pokora MJ, Widzowski DV (1992) Postnatal lead exposure induces supersensitivity to the stimulus properties of a D2-D3 agonist. Brain Res 598:162–172
Brockel BJ, Cory-Slechta DA (1999) Lead-induced decrements in waiting behavior: involvement of D2-like dopamine receptors. Pharmacol Biochem Behav 63:423–434
Lasley SM, Greenland RD, Minnema DJ, Michaelson IA (1984) Influence of chronic inorganic lead exposure on regional dopamine and 5-hydroxytryptamine turnover in rat brain. Neurochem Res 9:1675–1688
Kala SV, Jadhav AL (1995) Region-specific alterations in dopamine and serotonin metabolism in brains of rats exposed to low levels of lead. Neurotoxicology 16:297–308
Tyler CB, Galloway MP (1992) Acute administration of amphetamine: differential regulation of dopamine synthesis in dopamine projection fields. J Pharmacol Exp Ther 261:567–573
Elverfors A, Nissbrandt H (1992) Effects of d-amphetamine on dopaminergic neurotransmission; a comparison between the substantia nigra and the striatum. Neuropharmacology 31:661–670
Chetty CS, Reddy GR, Murthy KS, Johnson J, Sajwan K, Desaiah D (2001) Perinatal lead exposure alters the expression of neuronal nitric oxide synthase in rat brain. Int J Toxicol 20:113–120
Zhu DY, Lau L, Liu SH, Wei JS, Lu YM (2004) Activation of cAMP-response-element-binding protein (CREB) after focal cerebral ischemia stimulates neurogenesis in the adult dentate gyrus. Proc Natl Acad Sci USA 101:9453–9457
Acknowledgment
This study was supported by grant 2 PO5D 066 27 from the Ministry of Education and Science, Warsaw, Poland.
Author information
Authors and Affiliations
Corresponding author
Additional information
Special issue dedicated to Dr. Moussa Youdim.
Rights and permissions
About this article
Cite this article
Szczerbak, G., Nowak, P., Kostrzewa, R.M. et al. Maternal Lead Exposure Produces Long-Term Enhancement of Dopaminergic Reactivity in Rat Offspring. Neurochem Res 32, 1791–1798 (2007). https://doi.org/10.1007/s11064-007-9306-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-007-9306-0


