Abstract
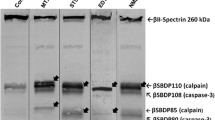
In the present study, we investigated chronological changes of μ-calpain, m-calpain and cleaved spectrin αII immunoreactivity in the ventral horn after transient spinal cord ischemia to investigate relationship between calpains and vulnerability to ischemia using abdominal aorta occlusion model in rabbits. Spinal cord sections at the level of L7 were immunostained with calpains and cleaved spectrin αII monoclonal antibodies. μ-Calpain and m-calpain immunoreactivity was significantly increased in the ischemic ventral horn at 30 min and 1 h after ischemia/reperfusion, respectively. Thereafter, they were decreased with time after ischemia/reperfusion: at 48 h after ischemia, their immunoreactivities nearly disappeared in the ischemic ventral horn. Cleaved spectrin αII immunoreactivity was significantly increased in the ventral horn of spinal cord at 12 h after ischemia/reperfusion, and thereafter, its immunoreactivity was decreased with time after ischemia/reperfusion. In addition, spectrin αII protein level (280 kDa) was decreased from 12 h after ischemia/reperfusion; in contrast, cleaved spectrin αII protein level (150 kDa) was significantly increased at 12 h after ischemia/reperfusion. In conclusion, our observations in this study indicate that calpain is associated with neuronal degeneration in the ventral horn at early time after transient spinal cord ischemia via the proteolysis of spectrin αII.






Similar content being viewed by others
References
Hayashi T, Sakurai M, Abe K, Sadahiro M, Tabayashi K, Itoyama Y (1998) Apoptosis of motor neurons with induction of caspases in the spinal cord after ischemia. Stroke 29:1007–1012
Kiyoshima T, Fukuda S, Matsumoto M, et al (2003) Lack of evidence for apoptosis as a cause of delayed onset paraplegia after spinal cord ischemia in rabbits. Anesth Analg 96:839–846
Brewer LA 3rd, Fosburg RG, Mulder GA, Verska JJ (1972) Spinal cord complications following surgery for coarctation of the aorta. A study of 66 cases. J Thorac Cardiovasc Surg 64:368–381
Crawford ES, Snyder DM, Cho GC, Roehm JO, Jr (1978) Progress in treatment of thoracoabdominal and abdominal aortic aneurysms involving celiac, superior mesenteric, and renal arteries. Ann Surg 188:404–422
Jarzem PF, Kostuik JP, Filiaggi M, Doyle DJ, Ethier R, Tator CH (1991) Spinal cord distraction: an in vitro study of length, tension, and tissue pressure. J Spinal Disord 4:177–182
Choi DW (1988) Calcium-mediated neurotoxicity: relationship to specific channel types and role in ischemic damage. Trends Neurosci 11:465–469
Siesjo BK (1981) Cell damage in the brain: a speculative synthesis. J Cerb Blood Flow Metab 1:155–185
Baudry M, Bundman M, Smith E, Lynch G (1981) Micromolar calcium stimulates proteolysis and glutamate binding in rat brain synaptic membranes. Science 212:937–938
Goll DE, Thompson VF, Li H, Wei W, Cong J (2003) The calpain system. Physiol Rev 83:731–801
Fukuda T, Adachi E, Kawashima S, Yoshiya I, Hashimoto PH (1990) Immunohistochemical distribution of calcium-activated neutral proteinases and endogenous CANP inhibitor in the rabbit hippocampus. J Comp Neurol 302:100–109
Lynch G (1998) Memory and the brain: unexpected chemistries and a new pharmacology. Neurobiol Learn Mem 70:82–100
Chan SL, Mattson MP (1999) Caspase and calpain substrates: roles in synaptic plasticity and cell death. J Neurosci Res 58:167–190
Carlson GD, Gorden C (2002) Current developments in spinal cord injury research. Spine J 2:116–128
Siman R, Baudry M, Lynch G (1984) Brain fodrin: substrate for calpain I, an endogenous calcium-activated protease. Proc Natl Acad Sci USA 81:3572–3576
Malik MN, Femko MD, Iqbal K, Wisniewski HM (1983) Purification and characterization of two forms of Ca2+-activated neutral protease from calf brain. J Biol Chem 258:8955–8962
Zimmerman UJP, Schlaepfer WW (1982) Characterization of a brain calcium-activated protease that degrades neurofilament proteins. Biochemistry 21:3977–3983
Chen MJ, Yap YW, Choy MS, et al (2006) Early induction of calpains in rotenone-mediated neuronal apoptosis. Neurosci Lett 397:69–73
Yoon S, Choi J, Huh JW, Hwang O, Kim D (2006) Calpain activation in okadaic-acid-induced neurodegeneration. Neuroreport 17:689–692
Czogalla A, Sikorski AF (2005) Spectrin and calpain: a ‘target’ and a ‘sniper’ in the pathology of neuronal cells. Cell Mol Life Sci 62:1913–1924
Li Z, Hogan EL, Banik NL (1996) Role of calpain in spinal cord injury: increased calpain immunoreactivity in rat spinal cord after impact trauma. Neurochem Res 21:441–448
Ray SK, Shields DC, Saido TC, et al (1999) Calpain activity and translational expression increased in spinal cord injury. Brain Res 816:375–380
Li M, Ona VO, Chen M, et al (2000) Functional role and therapeutic implications of neuronal caspase-1 and -3 in a mouse model of traumatic spinal cord injury. Neuroscience 99:333–342
Lee JC, Hwang IK, Park SK, et al (2005) Histochemical and electron microscopic study on motoneuron degeneration following transient spinal cord ischemia at normothermic conditions in rabbits. Anat Histol Embryol 34:252–257
Simpson RK, Jr, Robertson CS, Goodman JC (1990) Spinal cord ischemia-induced elevation of amino acids: extracellular measurement with microdialysis. Neurochem Res 15:635–639
Linn CP, Christensen BN (1992) Excitatory amino acid regulation of intracellular Ca in isolated catfish cone horizontal cells measured under voltage- and concentration-clamp conditions. J Neurosci 12:2156–2164
Sorimachi Y, Harada K, Saido TC, Ono T, Kawashima S, Yoshida K (1997) Downregulation of calpastatin in rat heart after brief ischemia and reperfusion. J Biochem (Tokyo) 122:743–748
Sakurai M, Nagata T, Abe K, Horinouchi T, Itoyama Y, Tabayashi K (2003) Survival and death-promoting events after transient spinal cord ischemia in rabbits: induction of Akt and caspase3 in motor neurons. J Thorac Cardiovasc Surg 125:370–377
Banik NL, Shields DC, Ray S, et al (1998) Role of calpain in spinal cord injury: effects of calpain and free radical inhibitors. Ann NY Acad Sci 844:131–137
Ray SK, Banik NL (2003) Calpain and its involvement in the pathophysiology of CNS injuries and diseases: therapeutic potential of calpain inhibitors for prevention of neurodegeneration. Curr Drug Targets CNS Neurol Disord 2:173–189
Bartus RT, Hayward NJ, Elliott PJ, et al (1994) Calpain inhibitor AK295 protects neurons from focal brain ischemia. Effects of postocclusion intra-arterial administration. Stroke 25:2265–2270
Markgraf CG, Velayo NL, Johnson MP, et al (1998) Six-hour window of opportunity for calpain inhibition in focal cerebral ischemia in rats. Stroke 29:152–158
Rami A, Agarwal R, Botez G, Winckler J (2000) mu-Calpain activation, DNA fragmentation, and synergistic effects of caspase and calpain inhibitors in protecting hippocampal neurons from ischemic damage. Brain Res 866:299–312
McCracken E, Hunter AJ, Patel S, Graham DI, Dewar D (1999) Calpain activation and cytoskeletal protein breakdown in the corpus callosum of head-injured patients. J Neurotrauma 16:749–761
Ray SK, Dixon CE, Banik NL (2002) Molecular mechanisms in the pathogenesis of traumatic brain injury. Histol Histopathol 17:1137–1152
Lee MS, Kwon YT, Li M, Peng J, Friedlander RM, Tsai LH (2000) Neurotoxicity induces cleavage of p35 to p25 by calpain. Nature 405:360–364
Saito K, Elce JS, Hamos JE, Nixon RA (1993) Widespread activation of calcium activated neutral proteinase (calpain) in the brain in Alzheimer disease: a potential molecular basis for neuronal degeneration. Proc Natl Acad Sci USA 90:2628–2632
Kakkar R, Raju RV, Sharma RK (1999) Calmodulin-dependent cyclic nucleotide phosphodiesterase (PDE1). Cell Mol Life Sci 55:1164–1186
Mouatt-Prigent A, Karlsson JO, Agid Y, Hirsch EC (1996) Increased M-calpain expression in the mesencephalon of patients with Parkinson’s disease but not in other neurodegenerative disorders involving the mesencephalon: a role in nerve cell death? Neuroscience 73:979–987
Emery E, Aldana P, Bunge MB, et al (1998) Apoptosis after traumatic human spinal cord injury. J Neurosurg 89:911–920
Banik NL, Shields DC, Ray SK, Hogan EL (1999) The pathophysiological role of calpain in spinal cord injury. In: Wang KKW, Yuen P (eds) Calpain pharmacology and toxicology of calcium dependent protease. Taylor and Francis, Philadelphia, pp 211–227
Lee JC, Hwang IK, Cho JH, et al (2004) Expression and changes of calbindin D-28k immunoreactivity in the ventral horn after transient spinal cord ischemia in rabbits. Neurosci Lett 369:145–149
Acknowledgments
The authors would like to thank Mr Suek Han, Seung Uk Lee and Ms Hyun Sook Kim for their technical help in this study. This work was supported by the MRC program of MOST/KOSEF (R13-2005-022-01002-0).
Author information
Authors and Affiliations
Corresponding author
Additional information
Jae-Chul Lee and In Koo Hwang equally contribute to this article.
Rights and permissions
About this article
Cite this article
Lee, JC., Hwang, I.K., Yoo, KY. et al. Degradation of Spectrin via Calpains in the Ventral Horn after Transient Spinal Cord Ischemia in Rabbits. Neurochem Res 31, 989–998 (2006). https://doi.org/10.1007/s11064-006-9104-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-006-9104-0