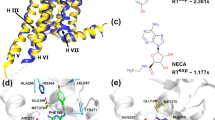
In our previous work [1], we have reconstructed the spatial structure of a full-size GABAB receptor using computer simulation. Considering the fact that baclofen is a selective agonist of this receptor, we attempted to search for binding sites of the molecule of this agent with the extracellular domain of a GABAB1 receptor subunit, assessed the molecular dynamics of their interaction, and calculated the energy of nonvalent interactions between the receptor and agonist molecule under study. A molecular docking approach used to estimate interactions between baclofen and the GABAB receptor extracellular domain allowed us to choose three sites for possible binding of the baclofen molecule to the receptor. Using molecular dynamics simulation, we identified two sites capable of providing stable coupling of baclofen with the GABAB receptor.
Similar content being viewed by others
Change history
11 August 2017
An erratum to this article has been published.
References
A. Yu. Nyporko, A. M. Naumenko, A. Golius, et al., “3D reconstruction of a full-size GABAB receptors,” Neurophysiology, 47, No. 5, 364-375 (2015).
N. G. Bowery and G. D. Pratt, “GABAB receptors as targets for drug action,” Arzneimittelforschung, 42, No. 2A, 215-223 (1992).
K. A. Jones, B. Borowsky, J. A. Tamm, et al., “GABAB receptors function as a heteromeric assembly of the subunits GABABR1 and GABABR2,” Nature, 396, 674-679 (1992).
K. Kaupmann, B. Malitschek, V. Schuler, et al., “GABAB receptor subtypes assemble into functional heteromeric complexes,” Nature, 396, 683-687 (1998).
J. H. White, A. Wise, M. J. Pain, et al., “Heterodimerization is required for the formation of a functional GABA(B) receptor,” Nature, 396, 679-682 (1998).
Y. Geng, M. Bush, L. Mosyak, et al., “Structural mechanism of ligand activation in human GABA(B) receptors,” Nature, 7479, No. 504, 254-259 (2013).
S. Kim, P. A. Thiessen, E. E. Bolton, et al., “PubChem substance and compound databases,” Nucl. Acids Res., 44, No. 1, 1202-1213 (2016).
Y. Wang, T. Suzek, J. Zhang, et al., “PubChem BioAssay: 2014 update,” Nucl. Acids Res., 42, No. 1, 1075-1082 (2014).
M. J. Frisch, G. W. Trucks, H. B. Schlegel, et al., Gaussian Revision, Wallingford, CT (2004).
V. Zoete, M. A. Cuendet, A. Grosdidier, and O. Michielin, “SwissParam, a fast force field generation tool for small organic molecules,” J. Comput. Chem., 11, No. 32, 2359-2368 (2011).
A. Grosdidier, V. Zoete, and O. Michielin, “SwissDock, a protein-small molecule docking web service based on EADock DSS,” Nucleic Acids Res., 39 (Web Server issue), W270-W277 (2011).
A. Grosdidier, V. Zoete, and O. Michielin, “Fast docking using the CHARMM force field with EADock DSS,” J. Comput. Chem., 32, No. 10, 2149-2159 (2011).
S. Pronk, S. Pall, R. Schulz, et al., “GROMACS 45: a high throughput and highly parallel open source molecular simulation toolkit,” Bioinformatics, 29, 845-854 (2013).
K. T. O’Neil and W. F. DeGrado, “A thermodynamic scale for the helix-forming tendencies of the commonly occurring amino acids,” Science, 250, 646-665 (1990).
R. S. Mulliken, “Electronic population analysis on LCAO-MO molecular wave functions,” J. Chem. Phys., 23, 1833-1840 (1955).
G. Costantino, A. Macchiarulo, A.-E. Guadix, and R. Pellicciari, “QSAR and molecular modeling studies of baclofen analogues as GABAB agonists. Insights into the role of the aromatic moiety in GABAB binding and activation,” J. Med. Chem., 44, 1827-1832 (2001).
T. C. Galvez, L. Prezeau, G. Milioti, et al., “Mapping the agonist binding site of GABAB type 1 subunit sheds light on the activation process of GABAB receptors,” J. Biol. Chem., 275, 411666-411674 (2000).
C. S. Cassidy, J. Lin, and P. A. Frey, “A new concept for the mechanisms of action of chymotrypsin: the role of the low-barrier hydrogen bond,” Biochemistry, 36, 4576-4584 (1997).
J. P. Gallivan and D. A. Dougherty, “A computational study of cation-π interactions vs salt bridges in aqueous medium. Implications for protein engineering,” J. Am. Chem. Soc., 122, 870-874 (2000).
R. Wintjens, J. Lievin, M. Rooman, and E. Buisine, “Contribution of cation-pi interactions to the stability of protein-DNA complexes,” Mol. Biol., 302, No. 2, 395-410 (2000).
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article is available at https://doi.org/10.1007/s11062-017-9651-9.
Rights and permissions
About this article
Cite this article
Naumenko, A.M., Shapoval, L.M., Nyporko, A.Y. et al. Computer Simulation of Molecular Interaction Between Baclofen and the GABAB Receptor. Neurophysiology 49, 2–7 (2017). https://doi.org/10.1007/s11062-017-9623-0
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11062-017-9623-0