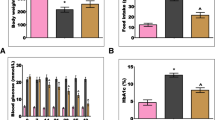
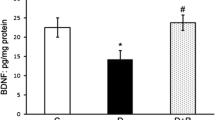
Reactive gliosis induced by metabolic disturbances and development of oxidative stress in the retina at diabetes mellitus is the key pathogenetic factor for the development of diabetic retinopathy. Fullerene C60 and some of its water-soluble derivates are known as rather potent antioxidants displaying neuroprotective capacities during the development of a number of pathologies and harmful influences on the organism. In our study, effects of nanostructures of hydrated C60 fullerene (C60HyFn) on the content and polypeptide composition of glial fibrillary acidic protein (GFAP) in the rat retina under conditions of streptozotocin (STZ)-induced diabetes were evaluated for the first time. Using immunoblotting, strong (more than twofold) up-regulation of GFAP expression in the diabetic rat retina, as compared with the control, was shown (P < 0.01). This was a result of activation of retinal glial cells induced by hyperglycemia. Increased GFAP immunolabeling that was indicative of reactive gliosis in the retina of diabetic rats was also confirmed immunohystochemically. Consumption of C60HyFn solution (60 nM) in drinking water by diabetic rats for 12 weeks caused a significant decrease in the GFAP level in comparison with that in untreated diabetic animals (P < 0.05). In addition, C60HyFn caused statistically significant lowering of the glycated hemoglobin concentration in blood serum of STZ-diabetic rats (P < 0.05). However, it exerted no effects on the insulin and glucose levels in blood of diabetic rats. Our results demonstrated that hydrated C60 fullerene as a potential high-efficacy retinoprotective agent within the initial period of diabetic retinopathy; it significantly suppresses activation of retinal macroglia.
Similar content being viewed by others
References
E. Rungger-Brändle, A. A. Dosso, and P. M. Leuenberger, “Glial reactivity, an early feature of diabetic retinopathy,” Invest. Ophthalmol. Vis. Sci., 41, No. 7, 1971-1980 (2000).
A. Ebneter and M. S. Zinkernagel, “Novelties in diabetic retinopathy,” Endocr. Dev., 31, 84–96 (2016).
R. Klein, B. E. K. Klein, and S. E. Moss, “Epidemiology of proliferative diabetic retinopathy,” Diabetes Care, 15, No. 12, 1875-1891 (1992).
B. Liu, X. Ma, D. Guo, et al., “Neuroprotective effect of alpha-lipoic acid on hydrostatic pressure-induced damage of retinal ganglion cells in vitro,” Neurosci. Lett., 526, No. 1, 24-28 (2012).
V. S. Nedzvetskii, G. A. Ushakova, S. G. Busygina, et al., “Effects of small doses of ionizing radiation on intermediate filaments and Ca2+-activated system of proteolysis in the rat brain,” Radiobiologiya, 31, No. 3, 333-339 (1991).
R. Dringen, P. G. Pawlowski, and J. Hirrlinger, “Peroxide detoxification by brain cells,” J. Neurosci. Res., 79, Nos. 1-2, 157-165 (2005).
M. A. Di Leo, G. Ghirlanda, N. Gentiloni Silveri, et al., “Potential therapeutic effect of antioxidants in experimental diabetic retina: a comparison between chronic taurine and vitamin E plus selenium supplementations,” Free Radic. Res., 37, No. 3, 323-330 (2003).
V. Nedzvetsky, G. Andrievsky, T. Chachibaia, and A. Tykhomyrov, “Differences in antioxidant/protective efficacy of hydrated C60 fullerene nanostructures in liver and brain of rats with streptozotocin-induced diabetes,” J. Diabetes Metab., 3, No. 8, 1-9 (2012).
G. Andrievsky, V. I. Bruskov, A. A. Tykhomyrov, and S. V. Gudkov, “Peculiarities of the antioxidant and radioprotective effects of hydrated C60 fullerene nanostuctures in vitro and in vivo,” Free Radical Biol. Med., 47, No. 6, 786-793 (2009).
J. Ryan, H. R. Bateman, A. Stover, et al., “Fullerene nanomaterials inhibit the allergic response,” J. Immunol., 179, No. 1, 665-672 (2007).
T. Mori, S. Ito, M. Namiki, et al., “Involvement of free radicals followed by the activation of phospholipase A2 in the mechanism that underlies the combined effects of methamphetamine and morphine on subacute toxicity or lethality in mice: Comparison of the therapeutic potential of fullerene, mepacrine, and cooling,” Toxicology, 236, No. 3, 149-157 (2007).
R. Bal, G. Türk, M. Tuzcu, et al., “Protective effects of nanostructures of hydrated C(60) fullerene on reproductive function in streptozotocin-diabetic male rats,” Toxicology, 282, No. 3, 69-81 (2011).
T. Baati, F. Bourasset, N. Gharbi, et al., “The prolongation of the lifespan of rats by repeated oral administration of [60]fullerene,” Biomaterials, 33, No. 19, 4936-4946 (2012).
M. M. Bradford, “A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding,” Anal. Biochem., 72, 248-254 (1976).
U. K. Laemmli, “Cleavage of structural proteins during the assembly of the head of bacteriophage T4,” Nature, 227, No. 5259, 680-685 (1970).
K. Canene-Adams, “Preparation of formalin-fixed paraffin-embedded tissue for immunohistochemistry,” Methods Enzymol., 533, 225-233 (2013).
G. V. Andrievsky, V. K. Klochkov, E. L. Karyakina, and N. O. Mchedlov-Petrossyan, “Studies of aqueous colloidal solutions of fullerene C60 by electron microscopy,” Chem. Phys. Lett., 300, Nos. 3-4, 392-396 (1999).
A. Tura, F. Schuettauf, P. P. Monnier, et al., “Efficacy of Rho-kinase inhibition in promoting cell survival and reducing reactive gliosis in the rodent retina,” Invest. Ophthalmol. Vis. Sci., 50, No. 1, 452-461 (2009).
M. Sugaya-Fukasawa, T. Watanabe, M. Tamura, et al., “Glial fibrillary acidic protein is one of the key factors underlying neuron-like elongation in PC12 cells,” Exp. Ther. Med., 2, No. 1, 85-87 (2011).
A. M. Joussen, S. Doehmen, M. L. Le, et al., “TNF-α mediated apoptosis plays an important role in the development of early diabetic retinopathy and long-term histopathological alterations,” Mol. Vis., 15, 1418-1428 (2009).
L. P. Aiello, A. Clermont, V. Arora, et al., “Inhibition of PKC by oral administration of ruboxistaurin is well tolerated and ameliorates diabetes-induced retinal hemodynamic abnormalities in patients,” Invest. Ophthalmol. Vis. Sci., 47, No. 1, 86-92 (2006).
V. Haurigot, P. Villacampa, A. Ribera, et al., “Increased intraocular insulin-like growth factor-I triggers bloodretinal barrier breakdown,” J. Biol. Chem., 284, No. 34, 22961-22969 (2009).
A. Hennis, S. Y. Wu, B. Nemesure, et al., “Hypertension, diabetes, and longitudinal changes in intraocular pressure ,” Ophthalmology, 110, No. 5, 908-914 (2003).
H. Zong, M. Ward, and A. W. Stitt, “AGEs, RAGE, and diabetic retinopathy,” Curr. Diabetes Rep., 11, No. 4, 244-252 (2011).
B. E. K. Klein, M. D. Knudtson, M. Y. Tsai, and R. Klein, “The relation of markers of inflammation and endothelial dysfunction to the prevalence and progression of diabetic retinopathy: Wisconsin epidemiologic study of diabetic retinopathy,” Arch. Ophthalmol., 127, No. 9, 1175-1182 (2009).
E. Lieth, A. J. Barber, B. Xu, et al., “Glial reactivity and impaired flutamate metabolism in short-term experimental diabetic retinopathy,” Diabetes, 47, No. 5, 815-820 (1998).
J. L. Wilkinson-Berka, “Angiotensin and diabetic retinopathy,” Int. J. Biochem. Cell Biol., 38, Nos. 5-6, 752-765 (2006).
W.-K. Ju, K-Y. Kim, Y. H. Noh, et al, “Increased mitochondrial fission and volume density by blocking glutamate excitotoxicity protect glaucomatous optic nerve head astrocytes,” Glia, 63, No. 5, 736-753 (2015).
P. Mamczur, B. Borsuk, J. Paszko, et al., “Astrocyteneuron crosstalk regulates the expression and subcellular localization of carbohydrate metabolism enzymes,” Glia, 63, No. 2, 328-340 (2015).
B. S. Ganesh and S. K. Chintala, “Inhibition of reactive gliosis attenuates excitotoxicity-mediated death of retinal ganglion cells,” PLoS One, 6, No. 3, e18305 (2011).
K. R. Hegde, S. Kovtun, and S. D. Varma, “Inhibition of glycolysis in the retina by oxidative stress: prevention by pyruvate,” Mol. Cell Biochem., 343, Nos. 1-2, 101-105 (2010).
L. Pellerin and P. J. Magistretti, “Sweet sixteen for ANLS,” J. Cerebr. Blood Flow Metab., 32, No. 7, 1152-1166 (2012).
M. V. Sofroniew, “Molecular dissection of reactive astrogliosis and glial scar formation,” Trends Neurosci., 32, No. 12, 638-647 (2009).
Q. J. Wang, “PKD at the crossroads of DAG and PKC signaling,” Trends Pharmacol. Sci., 27, No. 6, 317-323 (2006).
D. Koya and G. L. King, “Protein kinase C activation and the development of diabetic complications,” Diabetes, 47, No. 6, 859-866 (1998).
L. P. Aiello, S. E. Bursell, A. Clermont, et al., “Vascular endothelial growth factor-induced retinal permeability is mediated by protein kinase C in vivo and suppressed by an orally effective β-isoform-selective inhibitor,” Diabetes, 46, No. 9, 1473-1480 (1997).
Q. Li and D. G. Puro, “Diabetes-induced dysfunction of the glutamate transporter in retinal Muller cells,” Invest. Ophthalmol. Vis. Sci., 43, No. 9, 3109-3116 (2002).
G. Baydas, V. S. Nedzvetskii, S. V. Kirichenko, and P. A. Nerush, “Astrogliosis in the hippocampus and cortex and cognitive deficits in rats with streptozotocin-induced diabetes: effects of melatonin,” Neurophysiology, 40, No. 2, 91-97 (2008).
M. Ulas, C. Orhan, M. Tuzcu, et al., “Anti-diabetic potential of chromium histidinate in diabetic retinopathy rats,” BMC Complement. Alternat. Med., 15:16, 1-8 (2015).
A. A. Tykhomyrov, V. S. Nedzvetsky, V. K. Klochkov, and G. V. Andrievsky, “Nanostructures of hydrated C60 fullerene (C60HyFn) protect rat brain against alcohol impact and attenuate behavioral impairments of alcoholized animals,” Toxicology, 246, Nos. 2-3, 158-165 (2008).
S. Ye, T. Zhou, K. Cheng, et al., “Carboxylic acid fullerene (C60) derivatives attenuated neuroinflammatory responses by modulating mitochondrial dynamics,” Nanoscale Res. Lett., 10, No. 1, 953 (2015).
F. Fluri, D. Grünstein, E. Cam, et al., “Fullerenols and glucosamine fullerenes reduce infarct volume and cerebral inflammation after ischemic stroke in normotensive and hypertensive rats,” Exp. Neurol., 265, 142-151 (2015).
D. Giust, T. Da Ros, M. Martín, and J. L. Albasanz, “[60]Fullerene derivative modulates adenosine and metabotropic glutamate receptors gene expression: a possible protective effect against hypoxia,” J. Nanobiotechnol., 12, 27 (2014).
L. L. Dugan, L. Tian L, K. L. Quick, et al., “Carboxyfullerene neuroprotection postinjury in Parkinsonian nonhuman primates,” Ann. Neurol., 76, No. 3, 393-402 (2014).
E. G. Makarova, R. Y. Gordon, and I. Y. Podolski, “Fullerene C60 prevents neurotoxicity induced by intrahippocampal microinjection of amyloid-beta peptide,”J. Nanosci. Nanotechnol., 12, No. 1, 119-126 (2012).
A. G. Bobylev, N. V. Pen’kov, P. A. Troshin, and S. V. Gudkov, “Effect of dilution on aggregation of nanoparticles of polycarboxylic derivative of fullerene C60,” Biofizika, 60, No. 1, 38-43 (2015).
Y. Cheng, J. Qu, Y. Chen, et al., “Anterior segment neovascularization in diabetic retinopathy: a masquerade,” PLos One., 10, No. 6, e0123627 (2015).
I. Suárez, G. Bodega, M. Rubio, et al., “Astroglial induction of in vivo angiogenesis,” J. Neural. Transplant. Plast., 5, No. 1, 1-10 (1994).
N. J. Coorey, W. Shen, S. H. Chung, et al., “The role of glia in retinal vascular disease,” Clin. Exp. Optom., 95, No. 3, 266-281 (2012).
D. A. Antonetti, R. Klein, and T. W. Gardner, “Diabetic retinopathy,” New Engl. J. Med., 366, No. 13, 1227-1239 (2012).
B. A. Barres, “The mystery and magic of glia: a perspective on their roles in health and disease,” Neuron, 60, No. 3, 430-440 (2008).
S. M. Tan, D. Deliyanti, W. A. Figgett, et al., “Ebselen by modulating oxidative stress improves hypoxiainduced macroglial Müller cell and vascular injury in the retina,” Exp. Eye Res., 136, 1-8 (2015).
R. Pazdro and J. R. Burgess, “The role of vitamin E and oxidative stress in diabetes complications,” Mech. Ageing Dev., 131, No. 4, 276-286 (2010).
T. Jiang, Q. Chang, Z. Zhao, et al., “Melatonin-mediated cytoprotection against hyperglycemic injury in Müller cells,” PLoS One, 7, No. 12, e50661 (2012).
S. Yamagishi, S. Maeda, T. Matsui, et al., “Role of advanced glycation end products (AGEs) and oxidative stress in vascular complications in diabetes,” Biochim. Biophys. Acta, 1820, No. 5, 663-671 (2012).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Nedzvetskii, V.S., Pryshchepa, I.V., Tykhomyrov, A.A. et al. Inhibition of Reactive Gliosis in the Retina of Rats with Streptozotocin-Induced Diabetes under the Action of Hydrated C60 Fullerene. Neurophysiology 48, 130–140 (2016). https://doi.org/10.1007/s11062-016-9579-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11062-016-9579-5