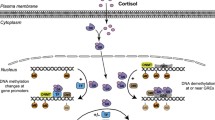
Abstract
Possible mechanisms of the effects exerted by stress (in the broad sense of the term) on the human genome and manifested in modifications of behavior are described in this review. Behavioral epigenetics opens new prospects for interpretation of the evolution of behavior induced by changes in living conditions. Epigenetic labels (imprints, methylation of DNA and/or covalent modifications of histones) appear under the influence of actual stress environmental influences, including social interactions. The appearance of such labels is not random; it is determined contextually and leads to behavioral disorders that can be transmitted through future generations. Stress phenomena of modern life, which are psychosocial in their nature, realize their effects via quite definite biological mechanisms. Epigenetic modifications are the most probable candidates for the role of relatively fast genetic mechanisms determining changes in behavior and mental health of great contingents of individuals living under contemporary conditions of ever-increasing stress loading.
Similar content being viewed by others
References
S. V. Faraone, M. T. Tsuang, and D. W. Tsuang, Genetics of Mental Disorders: A Guide for Students, Clinicians, and Researchers, Guilford Press, New York (1999).
R. Plomin, J. C. DeFries, G. E. McLean, and P. McGuffin, Behavioral Genetics, Worth Publ., New York (2008).
I. V. Ravich-Shcherbo, T. M. Malyutina, and Ye. L. Grigorenko, Psychogenetics, Aspekt-Press, Moscow (2002).
J. W. Gilger, “Contribution and promise of human behavioral genetics,” Human Biol., 72, No. 1, 229-255 (2000).
S. Torgersen, “Behavioral genetics of personality,” Current Psychiat. Rep., 7, No. 1, 51-56 (2005).
G. L. Engel, “The need for a new medical model: A challenge for biomedicine,” Science, 196, 129-136 (1997).
D. Pilgrim, “The biopsychosocial model in angloamerican psychiatry: Past, present and future,” J. Ment. Health, 11, No. 6, 585-594 (2002).
T. E. Moffitt, “Genetic and environmental influences in antisocial behaviors: evidence from behavioral-genetic research,” Adv. Genet., 55, 41-104 (2005).
Behavioral Genetics in the Postgenomic Era / R. Plomin, J. C. DeFries, I. W. Craig, and P. McGuffin (Eds.), Am. Psychol. Assoc., Washington, DC (2002).
Improving Health Systems and Services for Mental Health,World Health Organization (2009).
BMA Board of Science. Child and Adolescent Mental Health. A Guide for Healthcare Professionals, Br. Med. Assoc., London (2006).
S. Collishaw, B. Maughan, R. Goodman, et al., “Time trends in adolescent mental health,” J. Child Psychol. Psychiat., 45, No. 8, 1350-1362 (2004).
M. D. Golubovskii, Century of Genetics: Evolution of Ideas and Concepts, Borei Art, St. Petersburg (2000).
Z. Hochberg, R. Feil, M. Constancia, et al., “Child health, developmental plasticity, and epigenetic programming,” Endocrine Rev., 32, No. 2, 159-224 (2010).
A. M. Vaiserman, V. P. Voitenko, and L. V. Mekhova, “Epigenetic epidemiology of age-dependent diseases,” Ontogenez, 42, No. 1, 1-21 (2011).
Е. Jablonka and M. J. Lamb, “The inheritance of acquired epigenetic variations,” J. Theor. Biol., 139, No. 1, 69-83 (1989).
O. E. Landman, “The inheritance of acquired characteristics,” Annu. Rev. Genet., 25, 1-20 (1991).
E. J. Richards, “Inherited epigenetic variation – revisiting soft inheritance,” Nat. Rev. Genet., 7, 395-400 (2006).
D. L. Grodnitskii, “Epigenetic theory of evolution as a probable base for the new evolutionary synthesis,” Zh. Obshch. Biol., 62, No. 2, 99-109 (2001).
M. J. Meaney, M. Szyf, and J. R. Seckl, “Epigenetic mechanisms of perinatal programming of hypothalamic-pituitary-adrenal function and health,” Trends Mol. Med., 13, No. 7, 269-277 (2007).
J. M. Levenson, T. L. Roth, F. D. Lubin, et al., “Evidence that DNA (cytosine-5)methytransferase regulates synaptic plasticity in hippocampus,” J. Biol. Chem., 281, 15763-15773 (2006).
C. A. Miller and J. D. Sweat, “Covalent modification of DNA regulates memory function,” Neuron, 53, 857-869 (2007).
M. Szyf, “Epigenetic control of gene expression. The early life environment and the epigenome,” Biochim. Biophys. Acta (BBA), 1790, No. 9, 878-885 (2009).
M. Fagiolini, C. L. Jensen, and F. A. Champagne, “Epigenetic influences on brain development and plasticity,” Curr. Opin. Neurobiol., 19, No. 2, 207-212 (2009).
M. Szyf, “The dynamic epigenome and its implications in toxicology,” Toxicol. Sci., 100, 7-23 (2007).
P. D. Gluckman and M. A. Hanson, “Living in the past: evolution, development, and patterns of disease,” Science, 305, 1733-1736 (2004).
B. F. Vanyushin, S. G. Tkacheva, and A. N. Belozersky, “Rare bases in animal DNA,” Nature, 225, 948-949 (1970).
B. F. Vanyushin, “Methylation of DNA in cells of different organisms,” Usp. Sovrem. Biol., 77, No. 2, 68-90 (1974).
B. F. Vanyushin, N. A. Tushmalova, and L. V. Gus’kova, “Methylation of DNA in the brain as an index of involvement of the genome in mechanisms of individually acquired memory,” Dokl. Akad. Nauk. SSSR, 219, 742-744 (1974).
B. F. Vanyushin and Ye. B. Romanenko, “Changes of methylation of DNA in rats in ontogenesis and under the influence of hydrocortisone,” Biokhimiya, 44, 78-85 (1979).
B. F. Vanyushin, “Methylation of DNA and epigenetics,” Genetika, 42, No. 9, 1186-1199 (2006).
A. V. Prokhorchouk and A. S. Ruzov, “Methylation of the genome and its role in the functioning of an eukaryotic organism,” Genetika. 36, No. 11, 1475-1486 (2000).
R. Kumar and E. B. Thompson, “Gene regulation by the glucocorticoid receptor: structure/function relationship,” J. Steroid Biochem. Mol. Biol., 94, No. 5, 383-394 (2005).
M. Gehring, W. Reik, and S. Henikoff, “DNA demethylation by DNA repair,” Trends Genet., 25, 82-90 (2009).
B. E. Bernstein, A. Meissner, and E. S. Lander, “The mammalian epigenome,” Cell, 128, 669-681 (2007).
M. Szyf, “DNA methylation, the early-life social environment and behavioral disorders,” J. Neurodev. Disord., 3, No. 3, 238-249 (2011).
W. Fillipowicz, L. Jaskiewich, F. A. Kolb, and R. S. Pillai, “Post-transcriptional gene silencing by siRNAs and miRNAs,” Curr. Opin. Struct. Biol., 15, 331-341 (2005).
M. Tijsterman, R. F. Ketting, and R. H. Plasterk, “The genetics of DNA silencing,” Annu. Rev. Genet., 36, 489518 (2002).
A. Bilang-Bleuel, S. Ulbricht, Y. Chandramohan, et al., “Psychological stress increases histone H3 phosphorylation in adult dentate gyrus granule neurons: involvement in a glucocorticoid receptor-dependent behavioral response,” Eur. J. Neurosci., 22, No. 7, 16911700 (2005).
C. Tsigos and G. P. Chrousos, “Hypothalamic-pituitaryadrenal axis, neuroendocrine factors and stress,” J. Psychosom. Res., 53, No. 4, 865-871 (2002).
E. Charmandari, C. Tsigos, and G. P. Chrousos, “Endocrinology of the stress response,” Annu. Rev. Physiol., 67, 259-284 (2005).
G. Aguilera, A. Kiss, Y. Liu, and A. Kamitakahara, “Negative regulation of corticotropin releasing factor expression and limitation of stress response,” Stress, 10, No. 2, 153-161 (2007).
R. Hayashi, H. Wada, K. Ito, et al., “Effects of glucocorticoids on gene transcription,” Eur. J. Pharmacol., 500, Nos. 1/3, 51-62 (2004).
M. E. Bauer, “Stress, glucocorticoids and ageing of the immune system,” Stress, 8, No. 1, 69-83 (2005).
C. B. Nemeroff and W. W. Vale, “The neurobiology of depression: Inroads to treatment and new drugs discovery,” J. Clin. Psychiat., 66, Suppl. 6, 5-13 (2005).
K. Dedovic, A. Duchesne, J. Andrews, et al., “The brain and the stress axis: The neural correlates of cortisol regulation in response to stress,” NeuroImage, 47, 864871 (2009).
J. S. Snyder, A. Soumier, M. Brewer, et al., “Adult hippocampal neurogenesis buffers stress responses and depressive behavior,” Nature, 476, 458-461 (2011).
J. R. Seckl, “11β-hydroxysteroid dehydrogenase in the brain: a novel regulator of glucocorticoid action?” Front. Neuroendocrinol., 18, 49-99 (1997).
D. Wasserman, Depression. The Facts, Oxford Univ. Press, Oxford (2006).
L. Reba-Harrelson, A. Von Holle, R. M. Hamer, et al., “Patterns of maternal feeding and child eating associated with eating disorders in the Norwegian mother and child cohort study (MoBa),” Eat Behav., 11, No. 1, 54-61 (2010).
S. A. Swanson, S. J. Crow, D. Le Grange, et al., “Prevalence and correlates of eating disorders in adolescents. Results from the national comorbidity survey replication adolescent supplement,” Arch. Gen. Psychiat., 68, No. 7, 714-723 (2011).
C. N. Hales and D. J. P. Barker, “The thrifty phenotype hypothesis,” Br. Med. Bull., 60, 5-20 (2001).
K. A. Halmi, “Anorexia nervosa: An increasing problem in children and adolescents,” Dialogues Clin. Neurosci., 11, No. 1, 100-103 (2009).
V. A. Rozanov, Zh. K. Yemyasheva, and B, V, Biron, “Effect of a trauma in childhood in accumulation of stress events and formation of suicidal trends in the course of life,” Ukr. Med. Chasopys, 6, No. 86, 94-98 (2011).
S. J. Lupien, B. S. McEwen, M. R. Gunnar, et al., “Effects of stress throughout the lifespan on the brain, behavior and cognition,” Nature Rev. Neurosci., 10, No. 6, 434-445 (2009).
L. A. M. Welberg, J. R. Seckl, and M. C. Holmes, “Prenatal glucocorticoid programming of brain corticosteroid receptors and corticotropin-releasing hormone: Possible implication for behavior,” Neuroscience, 104, No. 1, 71-79 (2001).
J. I. Koenig, G. I. Elmer, P. D. Shepard, et al., “Prenatal exposure to a repeated variable stress paradigm elicits behavioral and neuroendocrinological changes in the adult offspring: Potential relevance to schizophrenia,” Behav. Brain Res., 156, No. 2, 251-261 (2005).
M. Weinstock, “The potential influence of maternal stress hormones on development and mental health of the offspring,” Brain Behav. Immunol., 19, No. 4, 296308 (2005).
N. S. Levitt, “Dexamethasone in the last week of pregnancy attenuates hippocampal glucocorticoid receptor gene expression and elevates blood pressure in the adult offspring of the rat,” Neuroendocrinology, 64, 412-418 (1996).
P. R. Lee, D. L. Brady, R. A. Shapiro, et al., “Prenatal stress generates deficits in rat social behavior: Reversal by oxytocin,” Brain Res., 1156, 152-167 (2007).
A. K. Kinnunen, J. I. Koenig, and G. Bilbe, “Repeated variable prenatal stress alters pre- and postsynaptic gene expression in the rat frontal pole,” J. Neurochem., 86, No. 3, 736-748 (2003).
A. G. Reznikov, N. D. Nosenko, L. V. Tarasenko, et al., “Early and long-term neuroendocrine effects of prenatal stress in male and female rats,” Neurosci. Behav. Physiol., 31, No. 1, 1-5 (2001).
A. G. Reznikov, V. P. Pishak, N. D. Nosenko, et al., Prenatal Stress and Neuroendocrinal Pathologies, Medakademiya, Chernovtsy (2004).
T. G. O’Connor, Y. Ben-Shlomo, J. Heron, et al., “Prenatal anxiety predicts individual differences in cortisol in preadolescent children,” Biol. Psychiat., 58, 211-217 (2005).
J. I. Koenig, C.-D. Walker, R. D. Romeo, et al., “Effects of stress across the lifespan,” Stress, 14, No. 5, 475-480 (2011).
T. Oberlander, J. Weinberg, M. Papsdorf, et al., “Prenatal exposure to maternal depression and methylation of human glucocorticoid receptor gene (NR3C1) in newborns,” Epigenetics, 3, 97-106 (2008).
A. M. Devlin, U. Brain, J. Austin et al., “Prenatal exposure to maternal depressed mood and the MTHFR C677T variant affect SLC6A4 methylation in infants at birth,” PLoS ONE, 5, No. 8, e12201. doi:10.1371/journal.pone.0012201 (2010).
R. Zh. Mukhamedrakhimov, A Mother and a Baby: Psychological Interaction, Rech’, Moscow (2003).
M. J. Meaney, “Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations,” Annu. Rev. Neurosci., 24, 1161-1192 (2001).
F. A. Champagne, “Epigenetic mechanisms and the transgenerational effects of maternal care,” Front. Neuroendocrinol., 29, 386-397 (2008).
D. Liu, J. Diorio, B. Tannenbaum, et al., “Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress,” Science, 277, No. 5332, 1659-1662 (1997).
I. C. G. Weaver, N. Cervoni, F. A. Champagne, et al., “Epigenetic programming by maternal behavior,” Nature Neurosci., 7, 847-854 (2004).
P. M. Plotsky and M. J. Meaney, “Early postnatal experience alters hypothalamic corticotropin-releasing factor (CRF) mRNA, median eminence CRF content and stress-induced release in adult rats,” Mol. Brain Res., 18, 195-200 (1993).
D. Liu, C. Caldji, S. Sharma, et al., “The effects of early life events on in vivo release of norepinephrine in the paraventricular nucleus of the hypothalamus and hypothalamic-pituitary-adrenal responses during stress,” J. Neuroendocrinol., 12, 5-12 (2000).
C. D. Walker, Z. Xu, J. Rochford, et al., “Naturally occurring variations in maternal care modulate the effects of repeated neonatal pain on behavioral sensitivity to thermal pain in the adult offspring,” Pain, 140, No. 1, 167-176 (2008).
C. D. Walker, “Maternal touch and feed as critical regulators of behavioral and stress responses in the offspring,” Dev. Psychobiol., 52, No. 7, 638-650 (2010).
S. V. Coutinho, P. M. Plotsky, M. Sablad, et al., “Neonatal maternal separation alters stress-induced responses to viscerosomatic nociceptive stimuli in rat,” Am. J. Physiol. Gastrointest. Liver Physiol., 282, G307-G316 (2002).
A. Caspi, K. Sugden, and T. E. Moffitt, “Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene,” Science, 301, 386-389 (2003).
E. B. Binder, R. G. Bradley, L. Wei, et al., “Association of FKBP5 polymorphisms and childhood abuse with risk of posttraumatic stress disorder symptoms in adults,” J. Am. Med. Assoc., 299, 1291-1305 (2008).
F. A. Champagne and J. P. Curley, “Epigenetic mechanisms mediating the long-term effects of maternal care on development,” Neurosci. Biobehav. Rev., 33, No. 4, 593-600 (2009).
D. Crews, “Epigenetics and its implications for behavioral neuroendocrinology,” Front. Neuroendocrinol., 29, No. 3, 344-357 (2008).
P. O. McGowan and M. Szyf, “The epigenetics of social adversity in early life: implications for mental health outcomes,” Neurobiol. Dis., 39, No. 1, 66-72 (2010).
B. Labonte and G. Turecki, “The epigenetics of suicide: explaining the biological effect of early life environmental adversity,” Arch. Suicide Res., 14, No. 4, 291-310 (2011).
P. O. McGowan, A. Sasaki, A. C. D’Alessio, et al., “Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse,” Nat. Neurosci., 12, No. 3, 342-348 (2009).
M. J. Meaney, J. Diorio, D. Francis, et al., “Postnatal handling increases the expression of cAMP-inducible transcription factors in the rat hippocampus: The effects of thyroid hormones and serotonin,” J. Neurosci., 20, No. 10, 3926-3935 (2000).
B. Buwalda, M. H. P. Kole, A. H. Veenema, et al., “Longterm effects of social stress on brain and behavior: a focus on hippocampal functioning,” Neurosci. Biobehav. Rev., 29, No. 1, 83-97 (2005).
N. M. Tsankova, O. Berton, W. Renthal, et al., “Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action,” Nat. Neurosci., 9, No. 4, 519-525 (2006).
R. S. Duman and L. M. Monteggia, “A neurotrophic model of stress-related mood disorders,” Biol. Psychiat., 59, No. 12, 1116-1127 (2006).
M. Sarchiapone, V. Carli, A. Roy, et al., “Association of polymorphism (Val66Met) of brain-derived neurotrophic factor with suicide attempts in depressed patients,” Neuropsychobiology, 57, No. 3, 139-145 (2008).
T. L. Roth, F. D. Lubin, A. J. Funk, et al., “Lasting epigenetic influence of early-life adversity on the BDNF gene,” Biol. Psychiat., 65, No. 9, 760-769 (2009).
S. Keller, M. Sarchiapone, F. Zarrilli, et al., “Increased BDNF promoter methylation in the Wernicke area of suicide subjects,” Arch. Gen. Psychiat., 69, No. 1, 62-70 (2012).
N. Borghol, M. Suderman, W. McArdle, et al., “Associations with early-life socio-economic position in adult DNA methylation,” Int. J. Epidemiol., 10.1093/ ije/dyr147 (2011).
J. Goodall, Chimpanzee in Nature: Behavior [in Russian], Mir, Moscow (1992).
L. A. Fairbanks, “Early experience and cross-generational continuity of mother-infant contact in vervet monkeys,” Dev. Psychobiol., 22, 669-681 (1986).
D. Mastripieri, K. Wallen, and K. A. Carrol, “Infant abuse runs in families of group-living pigtail macaques,” Child Abuse Negl., 21, 465-471 (1997).
D. Mastripieri, “Parenting styles of abusive mothers in group-living rhesus macaques,” Anim. Behav., 55, 1-11 (1998).
M. Bardi and M. A. Huffman, “Effects of maternal style on infant behavior in Japanese macaques (Macaca fuscata),” Dev. Psychobiol., 41, No. 4, 364-372 (2002).
D. Benoit and K. C. Parker, “Stability and transmission of attachment across three generations,” Child Dev., 65, 1444-1456 (1994).
M. H. van Ijzendoorn, “Adult attachment representations, parental responsiveness and infant attachment: a metaanalysis of the predictive validity of the adult attachment interview,” Psychol. Bull., 117, 387-403 (1995).
D. R. Pederson, K. E. Gleason, G. Moran, et al., “Maternal attachment representations maternal sensitivity and the infant-mother attachment relationship,” Dev. Psychol., 34, 925-933 (1998).
H. J. Lee, A. H. Macbeth, J. H. Pagani, and W. S. Young, “Oxytocin: the great facilitator of life,” Prog. Neurobiol., 88, No. 2, 127-151 (2009).
Z. R. Donaldson and L. J. Young, “Oxytocin, vasopressin, and the neurogenetics of sociality,” Science, 322, 900-904 (2008).
N. Numan, “Motivational system and the neural circuitry of maternal behavior in the rat,” Dev. Psychobiol., 49 , 12-21 (2007).
D. C. Francis, F. C. Champagne, and M. J. Meaney, “Variations in maternal behavior are associated with differences in oxytocin receptor levels in the rat,” J. Neuroendocrinol., 12, 1145-1148 (2000).
F. C. Champagne, J. Diorio, S. Sharma, et al., “Naturally occurring variations in maternal behavior in the rat are associated with differences in estrogen-inducible central oxytocin receptors,” Proc. Natl. Acad. Sci. USA, 98, 12736-12741 (2002).
F. A. Champagne, I. C. Weaver, J. Diorio, et al., “Maternal care associated with methylation of the estrogen receptor-alpha1b promoter and estrogen receptor-alpha expression in the medial preoptic area of female offspring,” Endocrinology, 147, 2909-2915 (2006).
J. P. Curley, F. A. Champagne, P. Bateson, et al., “Transgenerational effects of impaired maternal care on behavior of offspring and grandoffspring,” Anim. Behav., 75, No. 4, 1551-1561 (2008).
L. A. Smit-Rigter, F. A. Champagne, and J. A. van Hooft, “Lifelong impact of variations in maternal care on dendritic structure and function of cortical layer 2/3 pyramidal neurons in rat offspring,” PLoS ONE, 4, No. 4, e5167. doi: 10.1371/journal.pone.0005167 (2009).
E. Jablonka and G. Raz, “Transgenerational epigenetic inheritance: prevalence, mechanisms, and implications for the study of heredity and evolution,” Quart. Rev. Biol., 84, No. 2, 131-176 (2009).
M. E. Pembrey, L. O. Bygren, G. Kaati, et al., “Sexspecific, male-line transgenerational responses in humans,” Eur. J. Human Genet., 14, No. 2, 159-166 (2006).
C. Lindqvist, A. M. Janczak, D. Natt, et al., “Transmission of stress-induced learning impairment and associated brain gene expression from parents to offspring in chickens,” PLoS ONE, 2, No. 4, e364. doi: 10.1371/journal.pone.0000364 (2007).
Y. Liu, “Like father like son. A fresh review of the inheritance of acquired characteristics,” EMBO Rep., 8, No. 9, 798-803 (2007).
E. B. Keverne and J. P. Curley, “Epigenetics, brain evolution and behavior,” Front. Neuroendocrinol., 29, 398-412 (2008).
A. Joshi, “Behavior genetics in the post-genomics era: From genes to behavior and vice versa,” Curr. Sci., 89, No. 7, 1128-1135 (2005).
C. W. Kuzawa, “The fetal origins of developmental plasticity: are fetal clues reliable predictors of future nutritional environments?” Am. J. Human Biol., 17, 5-21 (2005).
B. M. Lester, E. Tronick, E. Nestler, et al., “Behavioral epigenetics,” Ann. N. Y. Acad. Sci., 1226, 14-33 (2011).
A. Caspi, J. McClay, T. E. Moffitt, et al., “Role of genotype in the cycle of violence in maltreated children,” Science, 297, 851-854 (2002).
J. Tabery, “Biometric and developmental gene-environment interactions: looking back, moving forward,” Dev. Psychopathol., 19, 961-976 (2007).
D. G. Kilpatrick, K. C. Koenen, K. J. Ruggiero, et al., “The serotonin transporter genotype and social support and moderation of posttraumatic stress disorder and depression in hurricane-exposed adults,” Am. J. Psychiat., 164, No. 11, 1693-1699 (2007).
T. Bradley, M. E. Cupples, and H. Irvine, “A case control study of a deprivation triangle: teenage motherhood, poor educational achievement and unemployment,” Int. J. Adolesc. Med. Health, 14, No. 2, 117-123 (2002).
E. Mittendorfer-Rutz, F. Rasmussen, and D. Wasserman, “Restricted fetal growth and adverse maternal psychosocial and socioeconomic conditions as risk factors for suicidal behavior of offspring: a cohort study,” Lancet, 364, 1135-1140 (2004).
G.-X. Jiang, F. Rasmussen, and D. Wasserman, “Short stature and poor psychological performance: risk factors for attempted suicide among Swedish make conscripts,” Acta Psychiat. Scand., 100, 433-440 (1999).
P. K. E. Magnusson, F. Rasmussen, D. A. Lawlor, et al., “Association of body mass index with suicide mortality: A prospective cohort study of more than one million men,” Am. J. Epidemiol., 163, 1-8 (2006).
C. S. Meade, T. S. Kershaw, and J. R. Ickovics, “The intergenerational cycle of teenage motherhood: an ecological approach,” Health Psychol., 27, No. 4, 419429 (2008).
J. D. Molina, F. Lopez-Munoz, D. J. Stein, et al., “Borderline personality disorder: A review and reformulation from evolutionary theory,” Med. Hypotheses, 73, 382-386 (2009).
K. S. Kendler, “Genetic and environmental pathways to suicidal behavior: Reflections of a genetic epidemiologist,” Eur. Psychiat., 25, 300–303 (2010).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rozanov, V.A. Epigenetics: Stress and Behavior. Neurophysiology 44, 332–350 (2012). https://doi.org/10.1007/s11062-012-9304-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11062-012-9304-y