Abstract
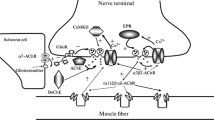
Results of biochemical and electrophysiological experiments allowing researchers to identify non-quantal release of acetylcholine (ACh), in addition to quantal release, from motor nerve endings, are discussed in the lecture. Based on the analysis of our own data and publications of other experimenters on the dependence of non-quantal secretion on the composition of the ion milieu, sensitivity of this phenomenon to different physiologically active compounds, and peculiarities of its temperature dependence, the authors conclude that non-quantal secretion of the transmitter is an active transport process, and not a passive leakage of ACh from the cytoplasm of the nerve terminal. It is hypothesized that a high-affinity system of choline uptake can play the role of the ACh carrier through the presynaptic membrane. The involvement of non-quantal release in the control of electrogenesis in the muscle fiber and the relation between processes of quantal and non-quantal secretion of the transmitter providing adequate functioning of the nerve terminal in the resting state and in the course of long-lasting high-frequency rhythmic activity are described. Data on the ability of glutamate and nitric oxide to selectively modulate this type of neurosecretion are analyzed. The possible role of non-quantal secretion of ACh in pathogenesis of intoxications of ACh esterase is discussed.
Similar content being viewed by others
References
W. Van der Kloot and J. Molgo, “Quantal acetylcholine release at the vertebrate neuromuscular junction,” Physiol. Rev., 74, 899–991 (1994).
W. Van der Kloot, “Loading and recycling of synaptic vesicles in the Torpedo electric organ and the vertebrate neuromuscular junction,” Prog. Neurobiol., 71, 269–303 (2003).
M. Israel and Y. Dunant, “Acetylcholine release. Reconstitution of the elementary quantal mechanism,” J. physiol., 92, 123–128 (1998).
R. L. Ruff, “Neurophysiology of the neuromuscular junction: overview,” Ann. N.Y. Acad. Sci., 998, 1–10 (2003).
S. J. Wood and C. R. Slater, “Safety factor at the neuromuscular junction,” Prog. Neurobiol., 64, 393–429 (2001).
A. L. Zefirov, “Vesicular cycle in the presynaptic nerve terminal,” Sechenov Ross. Fiziol. Zh., 93, 544–562 (2007).
J. F. Mitchell and A. Silver, “The spontaneous release of acetylcholine from the denervated hemidiaphragm of the rat,” J. Physiol., 165, 117–129 (1963).
P. Fletcher and T. Forrester, “The effect of curare on the release of acetylcholine from mammalian motor nerve terminals and an estimate of quantum content,” J. Physiol., 251, 131–144 (1975).
K. Krnjevic and D. W. Straughan, “The release of acetylcholine from the denervated rat diaphragm,” J. Physiol., 170, 371–378 (1964).
L. T. Potter, “Synthesis, storage and release of [14C]acetylcholine in isolated rat diaphragm muscles,” J. Physiol., 206, 145–166 (1970).
V. Dolezal and S. Tucek, “The synthesis and release of acetylcholine in normal and denervated rat diaphragms during incubation in vitro,” J. Physiol., 334, 461–474 (1983).
V. Dolezal and S. Tucek, “Effects of tetrodotoxin, Ca2+ absence, d-tubocurarine and vesamicol on spontaneous acetylcholine release from rat muscle,” J. Physiol., 458, 1–9 (1992).
P. C. Molenaar and R. L. Polak, “Acetylcholine synthesizing enzymes in frog skeletal muscle,” J. Neurochem., 35, 1021–1025 (1980).
S. Tucek, “The synthesis of acetylcholine in skeletal muscles of the rat,” J. Physiol., 322, 53–69 (1982).
B. Katz and R. Miledi, “Transmitter leakage from motor nerve endings,” Proc. Roy. Soc. Ser. B, 196, 59–72 (1977).
F. Vyskocil and P. Illes, “Non-quantal release of transmitter at mouse neuromuscular junction and its dependence on the activity of Na+-K+ ATP-ase,” Pflügers Arch., 370, 295–297 (1977).
F. Vyskocil, E. Nikolsky, and C. Edwards, “An analysis of the mechanisms underlying the non-quantal release of acetylcholine at the mouse neuromuscular junction,” Neuroscience, 9, 429–435 (1983).
C. Edwards, V. Dolezal, S. Tucek, et al., “A possible role for the acetylcholine transport system in non-quantal release of acetylcholine at the rodent myoneural junction,” Puerto Rico Health Sci. J., 7, 71–74 (1988).
F. Vyskocil and G. Vrbova, “Non-quantal release of acetylcholine affects polyneuronal innervation on developing rat muscle fibers,” Eur. J. Neurosci., 5, 1677–1683 (1993).
Y. L. Shih, “Abolishment of non-quantal release of acetylcholine from the mouse phrenic nerve endings by toosendanin,” Jpn. J. Physiol., 36, 601–605 (1986).
V. Dolezal, F. Vyskocil, and S. Tucek, “Decrease of the spontaneous non-quantal release of acetylcholine from the phrenic nerve in botulinum-poisoned rat diaphragm,” Pflügers Arch., 397, 319–322 (1983).
E. E. Nikolsky, V. A. Voronin, T. I. Oranska, and F. Vyskocil, “The dependence of non-quantal acetylcholine release on the choline-uptake system in the mouse diaphragm,” Pflügers Arch., 418, 74–78 (1991).
E. E. Nikolsky, T. I. Oranska, and F. Vyskocil, “Non-quantal acetylcholine release in the mouse diaphragm after phrenic nerve crush and during recovery,” Exp. Physiol., 81, 341–348 (1996).
D. C. Linden, M. W. Newton, A. D. Grinell, and D. J. Jenden, “Rapid decline in acetylcholine release and content of rat extensor longus muscle after denervation,” Exp. Neurol., 81, 613–626 (1983).
E. E. Nikolsky, T. I. Oranska, and F. Vyskocil, “Non-quantal acetylcholine release after cholinesterase inhibition in vivo,” Physiol. Res., 41, 333–334, (1992).
Y.-A. Sun and M.-m. Poo, “Non-quantal release of acetylcholine at a developing neuromuscular synapse in culture,” J. Neurosci., 5, 634–642 (1985).
J. Minic, J. Molgo, E. Karlsson, and E. Krejci, “Regulation of acetylcholine release by muscarinic receptors at the mouse neuromuscular junction depends on the activity of acetylcholinesterase,” Eur. J. Neurosci., 15, 439–448 (2002).
B. Katz and R. Miledi, “Does the motor nerve impulse evoke ‘non-quantal’ transmitter release?” Proc. Roy. Soc. Ser. B, 212, 131–137 (1981).
F. Vyskocil and P. Illes, “Electrophysiological examination of transmitter release in non-quantal form in the mouse diaphragm and the activity of membrane ATP-ase,” Physiol. Bohemoslov., 27, 449–455 (1978).
D. O. Smith, “Acetylcholine storage, release and leakage at the neuromuscular junction of mature adult and aged rats,” J. Physiol., 347, 161–176 (1984).
E. E. Nikolsky, V. A. Voronin, and F. Vyskocil, “Kinetic differences in the effect of calcium on quantal and non-quantal acetylcholine release at the murine diaphragm,” Neurosci. Lett., 123, 192–194 (1991).
H. Zemkov and F. Vyskocil, “Effect of Mg2+ on non-quantal acetylcholine release at the mouse neuromuscular junction,” Neurosci. Lett., 103, 293–297 (1989).
E. E. Nikol’skii and V. A. Voronin, “Temperature dependences of the processes of spontaneous quantal and non-quantal transmitter release from motor nerve endings of the mice,” Neirofiziologiya, 18, 361–367 (1986).
C. Edwards, V. Dolezal, S. Tucek, et al., “Is an acetylcholine transport system responsible for nonquantal release of acetylcholine at the rodent myoneural junction?” Proc. Natl. Acad. Sci. USA, 82, 3514–3518 (1985).
F. Vyskocil, “Inhibition of non-quantal acetylcholine leakage by 2(4-phenylpiperidine)cyclohexanol in the mouse diaphragm,” Neurosci. Lett., 59, 277–280 (1985).
J. J. Bray, J. W. Forrest, and J. I. Hubbard, “Evidence for the role of non-quantal acetylcholine in the maintenance of the membrane potential of rat skeletal muscle,” J. Physiol., 326, 285–296 (1982).
A. Urazaev, N. Naumenko, A. Malomough, et al., “Carbachol and acetylcholine delay the early postdenervation depolarization of muscle fibres through Mcholinergic receptors,” Neurosci. Res., 37, 255–263 (2000).
A. Kh. Urazaev, N. V. Naumenko, G. I. Poletaev, et al., “Acetylcholine and carbachol prevent muscle depolarization in denervated rat diaphragm,” NeuroReport, 8, 403–406 (1997).
R. A. Khairove, A. I. Malomouzh, N. V. Naumenko, and A. Kh. Urazayev, “Effect of oxotremorine on the resting membrane potential and cellular volume of rat muscle fibers with NO synthase blocked in vivo,” Byull. Éksp. Biol. Med., 135, 140–142 (2003).
F. Vyskocil, “Early postdenervation depolarization is controlled by acetylcholine and glutamate via nitric oxide regulation of the chloride transporter,” Neurochem. Res., 28, 575–585 (2003).
F. Vyskocil, “Action potentials of the rat diaphragm and their sensitivity to tetrodotoxin during postnatal development and old age,” Pflügers Arch., 352, 155–163 (1974).
E. E. Nikolsky, H. Zemkov, V. A. Voronin, and F. Vyskocil, “Role of non-quantal acetylcholine release in surplus polarization of mouse diaphragm fibers at the endplate zone,” J. Physiol., 477, 497–502 (1994).
F. Vyskocil, E. E. Nikolsky, H. Zemkov, and J. Krusek, “The role of non-quantal release of acetylcholine in regulation of postsynaptic membrane electrogenesis,” J. physiol., 89, 157–162 (1995).
I. I. Krivoi, E. V. Lopatina, and V. V. Kravtsove, “Roles of K+ channels and Na+, K+-ATPase in hyperpolarization of the membrane of skeletal muscle fibers evoked by acetylcholine,” Biol. Membr., 18, 10–15 (2001).
H. Dlouha, J. Teisinger, and F. Vyskocil, “Activation of membrane Na+/K+-ATPase of mouse skeletal muscle by acetylcholine and its inhibition by alpha-bungarotoxin, curare and atropine,” Pflü gers Arch., 380, 101–104 (1979).
L. Elfman, E. Heilbronn, and P. L. Jorgensen, “Fractionation of protein components of plasma membranes from the electric organ of Torpedo marmorata,” Biochim. Biophys. Acta, 693, 273–279 (1982).
P. Beguin, A. Beggah, S. Cotecchia, and K. Geering, “Adrenergic, dopaminergic, and muscarinic receptor stimulation leads to PKA phosphorylation of Na-K-ATPase,” Am. J. Physiol., 270, 131–137 (1996).
M. P. Danilenko, M. P. Panchenko, O. V. Yesirev, and V. A. Tkachuk, “Involvement of pertussis-toxin-sensitive G protein in muscarinic-receptor-mediated inhibition of K+-activated 4-nitrophenylphosphatase activity of cardiac sarcolemma,” Eur. J. Biochem., 194, 155–160 (1990).
M. Poo, J. W. Lam, and N. Orida, “Electrophoresis and diffusion in the plane of the cell membrane,” Biophysics, 26, 1–22 (1979).
S. Lin-Liu, W. R. Adey, and M. Poo, “Migration of cell surface concanavalin A receptors in pulsed electric fields,” Biophys. J., 45, 1211–1217 (1984).
R. A. Giniatullin, R. N. Khazipov, T. I. Oranska, et al., “The effect of non-quantal acetylcholine release on quantal miniature currents at mouse diaphragm,” J. Physiol., 466, 105–114 (1993).
D. P. Matyushkin, Functional Cellular Interactions in the Neuromuscular Apparatus [in Russian], Nauka, Leningrad (1980).
M. R. Mukhtarov, F. Vyskocil, A. K. Urazaev, and E. E. Nikolsky, “Non-quantal acetylcholine release is increased after nitric oxide synthase inhibition,” Physiol. Res., 48, 315–317 (1999).
M. R. Mukhtarov, A. Kh. Urazaev, E. E. Nikolsky, and F. Vyskocil, “Effect of nitric oxide and NO synthase inhibition on nonquantal acetylcholine release in the rat diaphragm,” Eur. J. Neurosci., 12, 980–986 (2000).
A. I. Malomouzh, M. R. Mukhtarov, E. E. Nikolsky, et al., “Glutamate regulation of non-quantal release of acetylcholine in the rat neuromuscular junction,” J. Neurochem., 85, 206–213 (2003).
A. I. Malomouzh, E. E. Nikolsky, E. M. Lieberman, et al., “Effect of N-acetylaspartylglutamate (NAAG) on non-quantal and spontaneous quantal release of acetylcholine at the neuromuscular synapse of rat,” J. Neurochem., 94, 257–267 (2005).
A. I. Malomouzh, M. R. Mukhtarov, E. E. Nikolsky, and F. Vyskocil, “Muscarinic M1 acetylcholine receptors regulate the non-quantal release of acetylcholine in the rat neuromuscular junction via NO-dependent mechanism,” J. Neurochem. (2007) [in press].
Z. Grozdanovic and H. G. Baumgarten, “Nitric oxide synthase in skeletal muscle fibers: a signaling component of the dystrophin-glycoprotein complex,” Histol. Histopathol., 14, 243–256 (1999).
J. S. Stamler and G. Meissner, “Physiology of nitric oxide in skeletal muscle,” Physiol. Rev., 81, 209–237 (2001).
A. I. Malomouzh, A. Kh. Urazayev, and E. E. Nikolskii, “Glutamatergic modulation of neuromuscular transmission in vertebrates,” Sechenov Ross. Fiziol. Zh., 90, 957–967 (2004).
A. V. Galkin, R. A. Giniatullin, M. R. Mukhtarov, et al., “ATP but not adenosine inhibits nonquantal acetylcholine release at the mouse neuromuscular junction,” Eur. J. Neurosci., 13, 2047–2053 (2001).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Neirofiziologiya/Neurophysiology, Vol. 39, Nos. 4/5, pp. 352–363, July–October, 2007.
Rights and permissions
About this article
Cite this article
Malomouzh, A.I., Nikol’skii, E.E. Non-quantal secretion of acetylcholine from motor nerve endings: Molecular mechanisms, physiological role, and regulation. Neurophysiology 39, 307–317 (2007). https://doi.org/10.1007/s11062-007-0042-5
Issue Date:
DOI: https://doi.org/10.1007/s11062-007-0042-5