Abstract
Purpose
Spontaneously occurring glioma in pet dogs is increasingly recognized as a valuable translational model for human glioblastoma. Canine high-grade glioma and human glioblastomas share many molecular similarities, including the accumulation of immunosuppressive regulatory T cells (Tregs) that inhibit anti-tumor immune responses. Identifying in dog mechanisms responsible for Treg recruitment may afford to target the cellular population driving immunosuppression, the results providing a rationale for translational clinical studies in human patients. Our group has previously identified C-C motif chemokine 2 (CCL2) as a glioma-derived T-reg chemoattractant acting on chemokine receptor 4 (CCR4) in a murine orthotopic glioma model. Recently, we demonstrated a robust increase of CCL2 in the brain tissue of canine patients bearing high-grade glioma.
Methods
We performed a series of in vitro experiments using canine Tregs and patient-derived canine glioma cell lines (GSC 1110, GSC 0514, J3T-Bg, G06A) to interrogate the CCL2-CCR4 signaling axis in the canine.
Results
We established a flow cytometry gating strategy for identifying and isolating FOXP3+ Tregs in dogs. The canine CD4 + CD25high T-cell population was highly enriched in FOXP3 and CCR4 expression, indicating they are bona fide Tregs. Canine Treg migration was enhanced by CCL2 or by glioma cell line-derived supernatant. Blockade of the CCL2-CCR4 axis significantly reduced migration of canine Tregs. CCL2 mRNA was expressed in all glioma cell lines, and expression increased when exposed to Tregs but not CD4 + helper T-cells.
Conclusion
Our study validates CCL2-CCR4 as a bi-directional Treg-glioma immunosuppressive and tumor-promoting axis in canine high-grade glioma.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Despite more than 450 NIH-sponsored phase II and III clinical trials, glioblastoma (GBM) remains a uniformly lethal primary brain tumor in adults [1,2,3,4]. Immunotherapeutic approaches that have advanced treatment in several non-CNS cancers have failed to show clinical benefit in GBM [5, 6]. This lack of progress highlights two key challenges restraining therapeutic advancement across GBM: (1) the microenvironment of adult GBM is markedly immunosuppressed, and (2) therapeutic successes in preclinical rodent models poorly translate into increased survival for human patients [7, 8]. The lack of therapeutic translation may rest, in part, on the homogeneous genetic backgrounds and lack of environmental immune influence of rodent models that do not faithfully recapitulate the heterogeneity and complexity of human tumors [8]. There is an urgent need for novel immunotherapeutic approaches to develop and test strategies to overcome the immunosuppressed microenvironment of GBM, as well as the incorporation of complementary preclinical models that more closely predict therapeutic efficacy in GBM patients [8].
While immunosuppression in GBM is multifactorial, the recruitment of CD4+CD25+FOXP3+ regulatory T cells (Tregs) inhibit anti-tumor immune responses and present a significant therapeutic challenge [9, 10]. We and others have previously shown that glioma-derived C-C Motif Chemokine Ligand 2 and 22 (CCL2 and CCL22) is a critical chemoattractant responsible for Treg recruitment into the GBM microenvironment. Moreover, increased intertumoral CCL2 expression correlated with reduced overall survival in human patients [9,10,11]. Using murine models, we determined that CCL2 induces Treg recruitment into the glioma microenvironment through the CC chemokine receptor type 4 (CCR4) expressed on Tregs. Importantly, targeting CCR4 reduced intertumoral Treg abundance and prolonged animal survival [9,10,11]. Therefore, the CCL2-CCR4 signaling axis may be a therapeutic target for GBM patients.
Canine high-grade gliomas (HGGs), which develop de novo in an outbred, immunocompetent host, are increasingly being pursued as a therapeutic model for the human GBM [12,13,14,15,16,17]. Canine HGGs, as defined by the Comparative Brain Tumor Consortium of the National Cancer Institute, encompass grade III and IV canine astrocytomas and oligodendrogliomas [12]. With a comparable disease incidence, naturally occurring canine HGGs share clinical, imaging, and histopathologic features with GBM [12]. Tregs have been identified in high-grade canine oligodendroglioma and astrocytoma [18, 19]. Moreover, we have demonstrated that CCL2 is robustly increased in canine HGG tumors relative to normal canine brain [16]. Notably, the CCL2-CCR4 axis is targetable and efficacious in canine patients. Blockade of CCR4 with the FDA-approved monoclonal antibody reduced tumoral Treg infiltration and improved survival time in dogs affected with bladder and prostate cancer [20, 21]. Small molecule inhibitors of CCR4 are also in development (AZD2098 and K777) [22, 23].
While Tregs have been observed in spontaneously occurring canine HGG, the mechanisms leading to their recruitment and function remain a critical knowledge gap for translational therapies targeting Tregs. Here, we performed a series of in vitro experiments using acutely isolated canine lymphocytes from healthy dogs and canine patient-derived glioma cell lines to interrogate the CCL2-CCR4 axis in dogs. Canine Tregs increased migration toward human recombinant CCL2 and glioma cell line-derived supernatant. However, this effect was abolished in the presence of CCL2 and CCR4 blockade. Moreover, canine glioma cells increased CCL2 mRNA expression when exposed to Tregs but not CD4 + helper T-cells. These data demonstrate that the CCL2-CCR4 signaling axis is necessary and sufficient for canine glioma cell-induced Treg migration and is an essential contributor to Treg recruitment to the tumor microenvironment in canine HGG. This work provides a mechanistic rationale for preclinical trials testing the efficacy of CCR4+Treg-targeted therapies in canine HGG.
Materials & methods
Isolation of canine T regulatory cells
This study was approved by the Institutional Animal Care and Use Committee and the UC Davis Veterinary Medical Teaching Hospital Clinical Trial Review Board. Three healthy client-owned dogs with a normal physical examination were recruited for this study. The cohort of blood donors consisted of a 2-year-old female spayed Labrador Retriever, a 2-year-old male neutered Labrador Retriever, and an 8-year-old male neutered Anatolian Shepherd mix. Following client consent, peripheral blood samples were taken from the jugular or lateral saphenous veins using standard veterinary technique and collected in ethylenediaminetetraacetic acid (EDTA) tubes. Blood samples were collected as necessary from each patient to complete a series of experiments described in this manuscript. Peripheral blood mononuclear cells (PBMCs) were isolated from whole blood by density gradient separation with Lymphoprep™ (Catalog #07851; Stemcell Technologies) and negative selection with ammonium chloride potassium (ACK) as previously described [24]. Purified cells were washed twice in PBS with 10% FBS by centrifuging at 600 g for five minutes at 4°C. After washing, cells were re-suspended in PBS with 10% FBS and prepared for fluorescence-activated cell sorting (FACS). Freshly isolated PBMCs were stained with mixtures of Pacific Blue (PB) conjugated anti-dog CD4 (clone YKIX302.9; Thermo Fischer Scientific) and Phycoerythrin (PE) conjugated anti-dog CD25 (clone P4A10; Thermo Fischer Scientific). Cells were washed twice with PBS at room temperature for 10 min prior to sorting with the Beckman Coulter “MoFlo Astrios EQ.” CD4 + cells were sorted by expression of CD25 into CD4 + CD25high and CD4 + CD25low populations. CD25 expression in the 90th percentile (highest 10%) was defined as CD4 + CD25 high, whereas CD25 expression at or below the 20th percentile (lowest 20%) was defined as CD4 + CD25low. Cells were immediately re-suspended in culture medium after sorting and seeded into 96-well plates at a concentration of 0.5-2 × 104 cells per well. Cells were cultured in 5% reduced serum 1640 RPMI for 8–10 h before downstream assays. Media contained RPMI-1640 complemented with 10% FBS, 10mM HEPES, 100 µg/mL streptomycin, 100U/mL penicillin, 2000U/ml recombinant IL-2 and 0.5mM β-mercaptoethanol.
Flow cytometry
Freshly isolated canine PBMCs underwent flow cytometry analysis using monoclonal antibodies (mAbs) against canine-specific or cross-reactive antigens. The following antibodies were utilized: CD3 (clone CA17.2A12; BioRad), CD4 (clone YKIX302.9; Thermo Fisher Scientific), CD8 (clone YCATE55.9, BioRad), CD25 (clone P4A10, Thermo Fisher Scientific), CCR4 (clone 1G1; BD Biosciences), and FOXP3 (clone FJK-16s; eBioscience). The viability of freshly isolated cells was assessed using live/dead fixable Near-IR cell staining (# L34976, Thermo Fisher Scientific).
FOXP3 visualization in CD4 + CD25high T lymphocytes
CD4+CD25high cells underwent FOXP3 intracellular staining with e-fluor 660 - conjugated anti-dog FOXP3 (clone FJK-16s; eBioscience). Intracellular staining, permeabilization, and fixation were performed using a commercially available transcription factor staining buffer set according to the manufacturer’s instructions (# 00-5523-00, eBioscience™). Cells were mounted with Vectashield with 4’,5-diamidino-2phenylindole (DAPI) (Vector Labs, Burlingame, CA, USA). Confocal imaging was performed on a Leica SP8 STED 3X microscope (objective 63x).
Canine glioma cell lines
In this study, we utilized four patient-derived canine high-grade astrocytoma cell lines (J3T-Bg, G06A, GSC 1110, and GSC 0514) [25, 26]. GSC 0514 and GSC 1110 lines are considered canine glioma stemcell-like cells and have been verified to express neural progenitor cell markers (SOX2, OLIG2, GFAP, NES) [25]. The genomic integrity of each cell line was verified by comparison of copy number alterations with parental tumor DNA using Illumina Canine HD SNP array and sequenced to confirm canine origin. All cell lines were routinely tested and confirmed to be mycoplasma-free by PCR. GSC1110 and GSC0514 cell lines were cultured in the serum-free conditions containing Neurobasal-A media (Thermo Fisher Scientific; 10,888,022), B27 supplement (Thermo Fisher Scientific; 12,587,010), N2 supplement (Thermo Fisher Scientific; 17,502,048), and 0.5X L-glutamine (Thermo Fisher Scientific; 25,030,081). bFGF (R&D Systems, Inc., Minneapolis, MN, USA; 233-FB-025), EGF (R&D Systems, Inc., USA; 236-EG-200), and PDGF (Gemini Bio-Products, West Sacramento, CA, USA; 300-178P) were added at 25 ng/ml concentration. J3T-Bg and G06A cell lines were cultured in media consisting of Dulbecco’s Modified Eagle Medium with high glucose and glutamine, supplemented with 10% heat-inactivated fetal calf serum, and 100 units/mL of penicillin, 100 µg/mL of streptomycin.
Transwell migration assays
The modified Boyden chamber assay was used to analyze Treg chemotaxis as previously described [9,10,11]. Tregs (3.1 × 104), suspended in 5% reduced serum 1640 RPMI media, were seeded into a 24-well plate with 5 μm pore polycarbonate filter inserts (Corning, Corning, NY). Bottom chambers contained (1) human recombinant CCL2 (rhCCL2; 0.5 ug/ml) in complete 1640 RPMI media, (2) J3TA or GSC 0514 cell supernatant obtained at 80% confluency, or (3) complete 1640 RPMI media only. Additional experiments were repeated following a 30-minute incubation of media with (1) anti-CCL2 antibody (5.0 ug/ml; clone # 280,702, R&D Systems) (rhCCL2 experiments only) or (2) pre-treatments of Tregs with CCR4 antagonist C 021 dihydrochloride (5 μm, Tocris). Following 6-hour incubation (37 °C, 5% CO2), cells in the bottom chamber were manually counted. Dead cells were excluded by trypan blue staining. Two independent experiments with technical duplicates per condition were performed.
Treg-glioma cell co-culture
Canine T lymphocytes (3.1 × 104; Tregs or CD4 + helper T cells), suspended in complete 1640 RPMI media, were seeded into a 24-well plate with 3 μm pore polycarbonate filter inserts (Corning, Corning, NY) and exposed to canine glioma cells (GSC 1110, GSC 0514, J3T-Bg, or G06A) at 80% confluency in the bottom chamber. Following 24-hour incubation (37 °C, 5% CO2), glioma cell lines were washed twice with PBS, and RNA was isolated as previously described [9,10,11].
Quantitative real-time PCR
Total RNA was isolated from glioma cells using the Direct-zol MiniPrep kit (Zymo Research, Irvine, CA, USA) according to the manufacturer’s instructions. DNase treatment was carried out on the column before RNA elution. Using one microgram of purified RNA, cDNA was reverse transcribed using the high-capacity cDNA reverse transcription kit (Thermo Fisher– Applied Biosystems, Waltham, MA, USA). We have used previously validated dog sets of primers [14, 20, 26]. Primer pair sequences were as follows: CCL2: 5′- GAGATCTGTGCTGACCCCAAA-3′ (forward) and 5′- TTGCAGTTTGGGTTTGGCTTT − 3′ (reverse); CCR4: 5′-CCC TAA GCC TTGCAC CAA AGA-3′ (forward) and 5′-TGT ACT TGA ACA GGA CCA CAA CCA-3′ (reverse); FOXP3: 5′ -GTCTTCGAGGAGCCAGAGGA-3′ (forward) and 5′ - GCACCCAGCTTCTCCTTCTC-3′ (reverse); GAPDH: 5′ TGTCCCCACCCCCAATGTATC-3′ (forward) and 5′-CTCCGATGCCTGCTTCACTACCTT-3′ (reverse). qPCR reactions consisted of primer pairs at a final concentration of 200nM, 50ng cDNA template, and SSoAdvanced Universal SYBR Green Supermix (Bio-Rad, Hercules, CA, USA) per manufacturer’s protocol. Reactions were carried out on a CFX connect machine (Bio-Rad) with a three-step cycle of 95ºC-15s, 60ºC-20s, and 72ºC-20s, followed by a melt curve ramp from 65ºC to 95ºC. Data were acquired during the 72ºC step and every 0.5ºC of the melt curve. All reactions were run as 20 µl triplicates for glioma cells or 20 µl duplicates for T-cells, and the average Cq was used as the data point for a given sample. Care was taken at each step to minimize assay variability: samples were processed in parallel; the same batch of reverse transcriptase was used for all samples. mRNA expression values were quantified by the 2-ΔΔCt method, whereby ΔCT = 18 S Ct– gene of interest Ct.
Statistical analysis
All statistical analyses were performed using GraphPad Prism 9 (GraphPad Software Inc., San Diego, CA). Data were tested for normality via the Shapiro-Wilks test. Data are reported as mean ± SEM. Statistical significance was assessed via unpaired two-tailed student’s t-test or ANOVA with Tukey’s multiple comparisons test. Results were regarded statistically significant for p < 0.05.
Results
High CD25 expression correlates with robust FOXP3 expression in CD4 + positive canine T lymphocytes
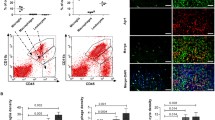
There is minimal data on identifying and isolating Tregs for downstream assays in dogs [27]. We hypothesized that freshly isolated canine CD4+CD25+ T cells would possess the FOXP3 transcription factor associated with the T regulatory cell, similar to human Tregs. To test this hypothesis, we assessed FOXP3 expression in acutely isolated CD4+CD25+ T cells from healthy dogs. When the total population of CD4+CD25+ cells was gated together, FOXP3 immunoreactivity was detected in 26.1% (33.4 ± 5.7, n = 3) of cells (Fig. 1A). However, when examining the expression of FOXP3 as a function of CD25 expression, we observed a positive correlation between CD25 expression and FOXP3 expression (Fig. 1B,C). The geometric mean fluorescence intensity (gMFI) of FOXP3 in the CD4+CD25high population was significantly increased compared to the CD4+CD25low and CD8+ cellular populations (p < 0.0001) (Fig. 1D). Furthermore, we observed a 17-fold increase in FOXP3 mRNA expression in CD4+CD25high cells compared to CD4+CD25low cells (p < 0.05) (Fig. 1E). Immunocytochemistry further confirmed intranuclear FOXP3 immunoreactivity in the CD4+CD25 high T cell population (Fig. 1F). Therefore, these data demonstrate that CD4+CD25high T cells are indeed FOXP3+ expressing cells, and positive selection of this population is a viable approach to isolate canine Tregs in the absence of intranuclear transcription factor FOXP3 immunostaining.
CD4 + CD25high lymphocytes are enriched with CCR4
Cellular CD25 expression was associated with enrichment of CCR4 expression on freshly isolated canine CD4 + lymphocytes (Fig. 2A). The proportion of cells expressing CCR4 was significantly increased in the CD4+CD25high population compared to the population of CD8+ cells (p < 0.05, n = 3) (Fig. 2B). CCR4 mRNA showed a 2.4-fold increase in CD4+CD25high cells compared to CD4+CD25low cells (p < 0.05, n = 3) (Fig. 2C). Thus, we positively selected CD4+CD25high lymphocytes, which express robust FOXP3 and are enriched for CCR4, to identify and isolate putative Treg cells for downstream assays.
The CCL2-CCR4 axis induces chemotaxis in canine Tregs
Freshly isolated canine Tregs (CD4+CD25high) were utilized to determine whether CCL2 is necessary and sufficient to induce canine Treg chemotaxis. Indeed, acutely isolated canine Tregs demonstrated robust chemotaxis toward human recombinant CCL2 (rhCCL2), with a 53.2% increase in migration relative to media alone (Fig. 3A; p = 0.0007). This effect was abolished with the addition of anti-CCL2 antibody (5.0 ug/ml) (Fig. 3B). Similarly, supernatant from GSC0514 (Fig. 3C; p < 0.0001) and J3T Bg (Fig. 3E; p < 0.0001) canine glioma cell lines induced a robust increase in Treg chemotaxis (32.2% and 54.03%, respectively), which was mitigated by addition of anti-CCL2 antibody (Fig. 3C, GSC0514 p = 0.006; Fig. 3E, J3T Bg p = 0.0001). Strikingly, Treg chemotaxis toward glioma cell supernatant was abolished following pre-treatment with CCR4 inhibitor (Fig. 3C, GSC0514 p < 0.0001; Fig. 3E, J3T Bg p < 0.0001), as well as combination treatment with an anti-CCL2 antibody and CCR4 inhibitor (Fig. 3C, GSC0514 p < 0.0001; Fig. 3E, J3T Bg p < 0.0001). CD4 + helper T cell chemotaxis was not affected by rhCCL2, GSC cell supernatant, nor perturbation of CCL2 or CCR4 signaling (Fig. 3D, p = 0.09). Chemotaxis of CD4 + helper T cell towards J3T Bg supernatant was minimally induced (8.06% increase) (Fig. 3F, p = 0.02), and this effect was mitigated by anti-CCL2 antibody (Fig. 3F, p = 0.01), CCR4 inhibitor (Fig. 3F, p = 0.03), and combination of both (Fig. 3F, p = 0.001). Taken together, these data support that the CCL2-CCR4 signaling axis is necessary and sufficient for canine Treg chemotaxis.
Canine tregs induce increased CCL2 mRNA expression in canine glioma cell lines
Glioma cells increase the secretion of CCL2 to recruit immune-regulatory cells in human glioma models [28]. To determine if canine Tregs can stimulate CCL2 expression in canine glioma cells, we performed co-culture experiments. Following 24-hour co-culture with Tregs, we observed increased CCL2 mRNA expression in each of four canine glioma cell lines (GSC0514: 0.95 ± 0.17- fold increased, 4A p < 0.001; J3T-Bg: 1.02 ± 0.33, Fig. 4B p < 0.05; GSC1110: 0.55 ± 0.11, Fig. 4C p < 0.1; and G06A: 0.56 ± 0.19-fold increase, Fig. 4D p < 0.05). mRNA levels of CCL2 were not significantly different when glioma cells were co-cultured with CD4 + helper T cells. (a) 0.08 ± 0.05- fold decrease in GSC0514, p = 0.8 (b) 0.30 ± 0.15 - fold increase in J3T-Bg, p = 0.6 (c) 0.47 ± 0.29- fold decrease in GSC1110, p = 0.2 and (d) 0.02 ± 0.1- fold decrease in G06A cell line, p = 0.9. (Fig. 4A, B, C, D).
Discussion
Spontaneously occurring glioma in pet dogs is increasingly recognized as a unique model with valuable translational potential. Canine preclinical research and clinical trials may allow fast-track clinical investigations in human patients [29,30,31]. Here, we have demonstrated that CCL2-CCR4 is a relevant, targetable, bi-directional Treg-glioma signaling axis in the dog. Our studies confirmed that canine glioma cells induced Treg chemotaxis through CCL2-CCR4 signaling, which was abolished following blockade. Given the marked immunosuppression induced by Tregs in the glioma microenvironment, targeting CCL2-CCR4 can potentially improve patient outcomes with existing conventional and other experimental glioma therapies. As CCR4 monoclonal antibodies are FDA-approved for treating Sézary syndrome, an aggressive human cutaneous T-cell lymphoma [32], clinical investigations in canine glioma patients can support studies in human glioblastoma patients.
Since the discovery of Tregs and their immunosuppressive, pro-cancerous properties, considerable effort has gone into defining, characterizing, and targeting murine and human Treg cells [33]. In recent years, the successful reversal of Treg-mediated immunosuppression has advanced approaches to human oncology [34,35,36,37]. Depleting or inhibiting intratumoral Treg influx in murine models of adenocarcinoma, lymphoma, colon carcinoma, melanoma, and thymoma reduced tumor growth and, in some cases, induced remission [34,35,36,37,38,39,40]. Phase 1 and II clinical trials investigating Treg-targeted therapies are currently underway in several cancer types [NCT03236129, NCT00986518, NCT05200559, NCT00847106, NCT02009384]. The work of several groups has shown that these immunosuppressive cells support gliomagenesis [38]. Blockade of Treg mobilization increases the survival of glioma-bearing mice, showing antineoplastic potential of targeting the recruitment capacity of these cells in glioblastoma [10, 11, 39, 40]. Canine Treg characterization is extremely limited [27]. Given the scarcity of information on the identification and isolation of canine Tregs, one of the objectives of our work was to establish selection criteria for the isolation of live dog Tregs to develop preclinical support for clinical studies in dogs and subsequently in human patients.
There is considerable overlap in surface marker expression between Tregs and helper T cells. Moreover, while overexpression and nuclear localization of the forkhead box transcription factor three (FOXP3) by CD4 + CD25 + T cells demonstrate high specificity for Tregs, detection requires cellular permeabilization and is unsuitable for isolating live Tregs. Similarly, as in human Tregs, we observed that the total population of CD4+CD25+ canine T cells harvested from freshly isolated PBMCs was poorly enriched in FOXP3 (Fig. 1A). However, cascaded gating via flow cytometry revealed a positive correlation between CD25 and FOXP3. Over 70% of these cells with the highest CD25 expression (90th percentile) also expressed FOXP3 (Fig. 1B). This gating strategy also proved to be a robust workflow for the selective isolation of canine CD4 + CD25 + expressing high levels of CCR4 and using the enriched Tregs for downstream assays from freshly isolated canine PBMCs. Wu et al., in their study, evaluated and confirmed the suppressive function of canine CD4 + CD25high T cells via proliferation assay [27]. Taken together, high CD25 expression is a critical marker for identifying canine Tregs and can serve as a surrogate marker for isolating these cells.
Our group and others have previously shown that glioma-derived CCL2 is a crucial chemoattractant responsible for Treg recruitment into the glioma microenvironment in murine models and human tissues [10, 11, 28]. An increased CCL2 expression has been correlated with reduced overall survival in human glioma patients [11]. Notably, antibody-mediated targeting of the Treg CCL2 high-affinity receptor, CCR4, reduced intertumoral Treg abundance and prolonged survival in a mouse glioma model [10, 11]. Our work also demonstrated that CCL2 is robustly increased in high-grade canine astrocytoma compared to normal brains [16] and given the role of CCL2 and CCR4 in both human and mouse models of gliomas, we studied here how this increased expression of CCL2 may affect the canine Tregs and CD4+ helper T-cells.
We performed a series of in vitro experiments to interrogate the CCL2-CCR4 axis in dogs. Importantly, canine Treg migration was significantly enhanced by canine glioma cell line-derived supernatant and later mitigated by an anti-CCL2 antibody. However, the most significant reduction in canine Treg migration was observed in the presence of a CCR4 antagonist or when combined with an anti-CCL2 antibody (dual blockade). This suggests that the CCR4 receptor may be the significant molecule mediating Treg migration to the tumor site in response to CCL2 and possibly other CCR4 ligands secreted in the tumor environment. In the veterinary literature, several other chemokines can bind and induce Treg migration via the CCR4 receptor. For instance, CCL17 was reported to bind the CCR4 receptor to trigger the migration of Tregs toward canine urothelial carcinoma [20]. These chemokines have yet to be the subject of our study, and we focus on interrogating the CCL2-CCR4 axis in canine gliomas as the next translational, targetable step of our work. A comprehensive evaluation of Treg-responsive chemokines within canine glioma supernatant and tumor microenvironment will be an essential next step to advance understanding of Treg biology in the context of canine high-grade glioma.
Here, we have interrogated the CCL2-CCR4 axis in canine glioma. Our data indicate that targeting either CCL2 or CCR4, alone or in combination, may represent a viable therapeutic strategy to diminish Treg accumulation in the glioma microenvironment. In the current literature, the blockade of either CCL2 or CCR4 was reported to have additional anti-tumor influence by targeting different than Treg subsets of pro-tumorigenic cells in various cancers. CCL2 and CCR4 or CCR2 have been proposed as a potential target for decreasing myeloid-derived suppressor cells (MDSCs) and tumor-associated macrophages (TAMs) recruitment in prostate cancer, breast cancer, malignant glioma, colorectal cancer, and lung carcinoma. [11, 41, 42].The availability of several therapeutic and experimental agents, including FDA-approved anti-CCR4 monoclonal antibody (Mogamulizumab) and small molecule inhibitors such as FLX475 [32, 43] AZD2098 and K777 [22, 23], facilitates preclinical trials in canines to determine safety, efficacy, and whether this approach may have adjunctive potential with other immunotherapies. Given the importance of host immune mechanisms in governing the response to immunotherapy, future canine clinical trials will be critical to inform therapeutic efficacy in human patients.In conclusion, we have established a workflow to identify and positively select canine Tregs from whole blood in dogs for downstream use. We have further established that the CCL2-CCR4 signaling axis is necessary and sufficient for canine Treg chemotaxis (Fig. 5). Importantly, this work demonstrates that canine HGG is a viable preclinical model for exploring immunotherapeutic approaches targeting this axis. Efficacy in canine patients will pave the way for promising clinical trials for human patients.
High CD25 expression correlates with robust FOXP3 expression in CD4+ positive canine T lymphocytes A) Representative flow cytometric plot illustrating a low percentage (26.1%) of FOXP3+ cells when gating includes all CD25+CD4+ cells. B) Representative flow cytometric plot illustrating a positive correlation between FOXP3 and CD25 expression in CD4+ canine T lymphocytes. In this example, FOXP3 expression was identified in 70.7% of cells with the highest CD25 expression (1.83%; red box). C) Scatter dot plot demonstrating the positive correlation between FOXP3 and CD25 expression. D) FOXP3 geometric mean fluorescence intensity and E) mRNA expression was significantly increased in CD4+ CD25high T lymphocytes compared to CD4+ CD25low and CD8+ T lymphocytes. F) Positive immunoreactivity for FOXP3 was observed co-localizing with nuclei of CD4+CD25high T lymphocytes. Three biological replicates were tested (n=3 dogs) with three technical replicates per sample. Comparisons are based on one-way ANOVA with Tukey’s multiple comparisons test and unpaired two-tailed student’s t-test, where bars represent the group mean with the standard error of the mean (SEM). *p<0.05
Canine Tregs has the highest expression of CCR4 amongst T-cells. A) Representative flow cytometric plot illustrating the percentage of CCR4+ cells within CD4+CD25+ and CD8+ canine T lymphocytes. In this example, CCR4 expression was identified in 28.5% of CD4+ CD25high cells and 8.6% of CD4+ CD25low cells. The expression of CCR4 in CD8+ T lymphocytes was minimal, 1.71%. B) The percentage of CCR4+ cells identified via flow cytometry and C) mRNA expression was significantly increased in CD4+CD25high T lymphocytes (2.43-fold increase) compared to CD4+CD25low and CD8+ T lymphocytes. Three biological replicates were tested (n=3 dogs) with three technical replicates per sample. Comparisons are based on one-way ANOVA with Tukey’s multiple comparisons test and unpaired two-tailed student’s t-test, where bars represent the group mean with standard error of the mean (SEM). *p<0.05
The CCL2-CCR4 axis induces chemotaxis in canine Tregs. A) Acutely isolated canine Tregs demonstrated robust chemotaxis toward human recombinant CCL2 (rhCCL2; 0.5 ug/ml) relative to media alone (p=0.0007). This effect was normalized by adding anti-CCL2 antibody (5.0 ug/ml; p=0.15). B) Chemotaxis of CD4+ helper T-cells was not affected by rhCCL2 (p=0.2) or anti-CCL2 antibody (p=0.4). C) Chemotaxis of acutely isolated canine Tregs (CD4+CD25high) increased toward GSC0514 derived supernatant (p<0.0001) and E) J3T Bg derived supernatant (p<0.0001). This effect was mitigated with the addition of anti CCL2 antibody (GSC0514, p=0.006; J3T Bg, p=0.0001) and abolished following Treg pre-treatment with CCR4 inhibitor (GSC0514 p<0.0001; J3T Bg p<0.0001) and a combination of anti-CCL2 and CCR4 inhibition (GSC0514 p<0.0001; J3T Bg p<0.0001). Migration of CD4+ helper T-cells was not affected by D) GSC0514 derived supernatant (p=0.06) nor perturbation of CCL2 (p=0.8), CCR4 (p=0.8) vs. combination of the two signaling (p=0.09). Chemotaxis of CD4+ helper T-cells towards J3T Bg supernatant was induced, F) (p=0.02), and this effect was mitigated by anti-CCL2 antibody (p=0.016); CCR4 inhibitor (p=0.03) and combination of both (p=0.001). Two independent experiments with technical duplicates per each condition were performed. Comparisons are based on one-way ANOVA with Tukey’s multiple comparisons test; bars represent the group mean with the standard error of the mean (SEM). ****p<0.0001
Canine glioma CCL2 mRNA expression increases when exposed to Tregs but not conventional T-cells. mRNA levels of CCL2 were increased in A) GSC0514, 0.95 ± 0.17- fold increased, p<0.001; B) J3T Bg, 1.02 ± 0.33- fold increased, p<0.05; C) GSC0111, 0.55 ± 0.11-fold increase, p<0.1; D) G06A, 0.56 ± 0.19-fold increase, p<0.05, co-cultured with CD4+CD25high T lymphocytes relative to GSC0514, J3T Bg, GSC0111 and G06A cell lines without exposure to CD4+CD25high. mRNA levels of CCL2 were not significantly different when glioma cells were co-cultured with conventional T-cells. A) 0.08 ± 0.05- fold decrease in GSC0514, p=0.8 B) 0.30 ± 0.15 - fold increase in J3T-Bg, p=0.6 C) 0.47 ± 0.29- fold decrease in GSC1110, p=0.2 and D) 0.02 ± 0.1- fold decrease in G06A cell line, p=0.9. All reactions were run as 20μl triplicates per each condition. Comparisons are based on one-way ANOVA with Tukey’s multiple comparisons test; bars represent the group mean with the standard error of the mean (SEM). ****p<0.0001
Data availability
No datasets were generated or analysed during the current study.
References
Stupp R, Hegi ME, Gorlia T, Erridge SC, Perry J, Hong YK, Aldape KD, Lhermitte B, Pietsch T, Grujicic D, Steinbach JP, Wick W, Tarnawski R, Nam DH, Hau P, Weyerbrock A, Taphoorn MJ, Shen CC, Rao N, Thurzo L, Herrlinger U, Gupta T, Kortmann RD, Adamska K, McBain C, Brandes AA, Tonn JC, Schnell O, Wiegel T, Kim CY, Nabors LB, Reardon DA, van den Bent MJ, Hicking C, Markivskyy A, Picard M, Weller M, team Cs (2014) European Organisation for R, Treatment of C, Canadian Brain Tumor C, Cilengitide combined with standard treatment for patients with newly diagnosed glioblastoma with methylated MGMT promoter (CENTRIC EORTC 26071– 22072 study): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol 15: 1100–1108 https://doi.org/10.1016/S1470-2045(14)70379-1
Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO, European Organisation for R, Treatment of Cancer Brain T, Radiotherapy G, National Cancer Institute of Canada Clinical Trials G (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352: 987–996 https://doi.org/10.1056/NEJMoa043330
Gilbert MR, Wang M, Aldape KD, Stupp R, Hegi ME, Jaeckle KA, Armstrong TS, Wefel JS, Won M, Blumenthal DT, Mahajan A, Schultz CJ, Erridge S, Baumert B, Hopkins KI, Tzuk-Shina T, Brown PD, Chakravarti A, Curran WJ Jr., Mehta MP (2013) Dose-dense temozolomide for newly diagnosed glioblastoma: a randomized phase III clinical trial. J Clin Oncol 31:4085–4091. https://doi.org/10.1200/JCO.2013.49.6968
Westphal M, Heese O, Steinbach JP, Schnell O, Schackert G, Mehdorn M, Schulz D, Simon M, Schlegel U, Senft C, Geletneky K, Braun C, Hartung JG, Reuter D, Metz MW, Bach F, Pietsch T (2015) A randomised, open label phase III trial with nimotuzumab, an anti-epidermal growth factor receptor monoclonal antibody in the treatment of newly diagnosed adult glioblastoma. Eur J Cancer 51:522–532. https://doi.org/10.1016/j.ejca.2014.12.019
Panek WK, Kane JR, Young JS, Rashidi A, Kim JW, Kanojia D, Lesniak MS (2017) Hitting the nail on the head: combining oncolytic adenovirus-mediated virotherapy and immunomodulation for the treatment of glioma. Oncotarget 8:89391–89405. https://doi.org/10.18632/oncotarget.20810
Lee-Chang C, Miska J, Hou D, Rashidi A, Zhang P, Burga RA, Jusue-Torres I, Xiao T, Arrieta VA, Zhang DY, Lopez-Rosas A, Han Y, Sonabend AM, Horbinski CM, Stupp R, Balyasnikova IV, Lesniak MS (2021) Activation of 4-1BBL + B cells with CD40 agonism and IFNgamma elicits potent immunity against glioblastoma. J Exp Med 218. https://doi.org/10.1084/jem.20200913
Raychaudhuri B, Rayman P, Ireland J, Ko J, Rini B, Borden EC, Garcia J, Vogelbaum MA, Finke J (2011) Myeloid-derived suppressor cell accumulation and function in patients with newly diagnosed glioblastoma. Neuro Oncol 13:591–599. https://doi.org/10.1093/neuonc/nor042
Slika H, Karimov Z, Alimonti P, Abou-Mrad T, De Fazio E, Alomari S, Tyler B (2023) Preclinical models and technologies in Glioblastoma Research: evolution, current state, and future avenues. Int J Mol Sci 24. https://doi.org/10.3390/ijms242216316
Miska J, Lee-Chang C, Rashidi A, Muroski ME, Chang AL, Lopez-Rosas A, Zhang P, Panek WK, Cordero A, Han Y, Ahmed AU, Chandel NS, Lesniak MS (2019) HIF-1alpha is a metabolic switch between Glycolytic-Driven Migration and oxidative phosphorylation-driven immunosuppression of Tregs in glioblastoma. Cell Rep 27:226–237e224. https://doi.org/10.1016/j.celrep.2019.03.029
Panek WK, Pituch KC, Miska J, Kim JW, Rashidi A, Kanojia D, Lopez-Rosas A, Han Y, Yu D, Chang CL, Kane JR, Zhang P, Cordero A, Lesniak MS (2019) Local application of autologous platelet-rich fibrin patch (PRF-P) Suppresses Regulatory T Cell Recruitment in a murine glioma model. Mol Neurobiol 56:5032–5040. https://doi.org/10.1007/s12035-018-1430-0
Chang AL, Miska J, Wainwright DA, Dey M, Rivetta CV, Yu D, Kanojia D, Pituch KC, Qiao J, Pytel P, Han Y, Wu M, Zhang L, Horbinski CM, Ahmed AU, Lesniak MS (2016) CCL2 produced by the glioma microenvironment is essential for the recruitment of regulatory T cells and myeloid-derived suppressor cells. Cancer Res 76:5671–5682. https://doi.org/10.1158/0008-5472.CAN-16-0144
Koehler JW, Miller AD, Miller CR, Porter B, Aldape K, Beck J, Brat D, Cornax I, Corps K, Frank C, Giannini C, Horbinski C, Huse JT, O’Sullivan MG, Rissi DR, Mark Simpson R, Woolard K, Shih JH, Mazcko C, Gilbert MR, LeBlanc AK (2018) A revised diagnostic classification of canine glioma: towards validation of the Canine Glioma patient as a naturally occurring preclinical model for human glioma. J Neuropathol Exp Neurol 77:1039–1054. https://doi.org/10.1093/jnen/nly085
Mariani CL, Schubert TA, House RA, Wong MA, Hopkins AL, Barnes Heller HL, Milner RJ, Lester NV, Lurie DM, Rajon DA, Friedman WA, Bova FJ (2015) Frameless stereotactic radiosurgery for the treatment of primary intracranial tumours in dogs. Vet Comp Oncol 13:409–423. https://doi.org/10.1111/vco.12056
Toedebusch RG, Wei NW, Simafranca KT, Furth-Jacobus JA, Brust-Mascher I, Stewart SL, Dickinson PJ, Woolard KD, Li CF, Vernau KM, Meyers FJ, Toedebusch CM (2023) Intra- and intertumoral microglia/macrophage infiltration and their associated molecular signature is highly variable in canine oligodendroglioma: a preliminary evaluation. Vet Sci 10. https://doi.org/10.3390/vetsci10060403
Amin SB, Anderson KJ, Boudreau CE, Martinez-Ledesma E, Kocakavuk E, Johnson KC, Barthel FP, Varn FS, Kassab C, Ling X, Kim H, Barter M, Lau CC, Ngan CY, Chapman M, Koehler JW, Long JP, Miller AD, Miller CR, Porter BF, Rissi DR, Mazcko C, LeBlanc AK, Dickinson PJ, Packer RA, Taylor AR, Rossmeisl JH Jr., Woolard KD, Heimberger AB, Levine JM, Verhaak RGW (2020) Comparative molecular life history of spontaneous canine and human gliomas. Cancer Cell 37: 243–257 e247 https://doi.org/10.1016/j.ccell.2020.01.004
Toedebusch R, Grodzki AC, Dickinson PJ, Woolard K, Vinson N, Sturges B, Snyder J, Li CF, Nagasaka O, Consales B, Vernau K, Knipe M, Murthy V, Lein PJ, Toedebusch CM (2021) Glioma-associated microglia/macrophages augment tumorigenicity in canine astrocytoma, a naturally occurring model of human glioma. Neurooncol Adv 3:vdab062. https://doi.org/10.1093/noajnl/vdab062
Boudreau CE, York D, Higgins RJ, LeCouteur RA, Dickinson PJ (2017) Molecular signalling pathways in canine gliomas. Vet Comp Oncol 15:133–150. https://doi.org/10.1111/vco.12147
Pi Castro D, Jose-Lopez R, Fernandez Flores F, Rabanal Prados RM, Mandara MT, Arus C, Pumarola Batlle M (2020) Expression of FOXP3 in canine gliomas: immunohistochemical study of tumor-infiltrating regulatory lymphocytes. J Neuropathol Exp Neurol 79:184–193. https://doi.org/10.1093/jnen/nlz120
Krane GA, O’Dea CA, Malarkey DE, Miller AD, Miller CR, Tokarz DA, Jensen HL, Janardhan KS, Shockley KR, Flagler N, Rainess BA, Mariani CL (2021) Immunohistochemical evaluation of immune cell infiltration in canine gliomas. Vet Pathol 58:952–963. https://doi.org/10.1177/03009858211023946
Maeda S, Murakami K, Inoue A, Yonezawa T, Matsuki N (2019) CCR4 blockade depletes regulatory T cells and prolongs survival in a canine model of bladder cancer. Cancer Immunol Res 7:1175–1187. https://doi.org/10.1158/2326-6066.CIR-18-0751
Maeda S, Motegi T, Iio A, Kaji K, Goto-Koshino Y, Eto S, Ikeda N, Nakagawa T, Nishimura R, Yonezawa T, Momoi Y (2022) Anti-CCR4 treatment depletes regulatory T cells and leads to clinical activity in a canine model of advanced prostate cancer. J Immunother Cancer 10. https://doi.org/10.1136/jitc-2021-003731
Kindon N, Andrews G, Baxter A, Cheshire D, Hemsley P, Johnson T, Liu YZ, McGinnity D, McHale M, Mete A, Reuberson J, Roberts B, Steele J, Teobald B, Unitt J, Vaughan D, Walters I, Stocks MJ (2017) Discovery of AZD-2098 and AZD-1678, two potent and bioavailable CCR4 receptor antagonists. ACS Med Chem Lett 8:981–986. https://doi.org/10.1021/acsmedchemlett.7b00315
Sato T, Iwase M, Miyama M, Komai M, Ohshima E, Asai A, Yano H, Miki I (2013) Internalization of CCR4 and inhibition of chemotaxis by K777, a potent and selective CCR4 antagonist. Pharmacology 91:305–313. https://doi.org/10.1159/000350390
Riedhammer C, Halbritter D, Weissert R (2016) Peripheral blood mononuclear cells: isolation, freezing, thawing, and culture. Methods Mol Biol 1304:53–61. https://doi.org/10.1007/7651_2014_99
Rainov NG, Koch S, Sena-Esteves M, Berens ME (2000) Characterization of a canine glioma cell line as related to established experimental brain tumor models. J Neuropathol Exp Neurol 59:607–613. https://doi.org/10.1093/jnen/59.7.607
Porcellato I, Brachelente C, Cappelli K, Menchetti L, Silvestri S, Sforna M, Mecocci S, Iussich S, Leonardi L, Mechelli L (2021) FoxP3, CTLA-4, and IDO in canine melanocytic tumors. Vet Pathol 58:42–52. https://doi.org/10.1177/0300985820960131
Wu Y, Chang YM, Stell AJ, Priestnall SL, Sharma E, Goulart MR, Gribben J, Xia D, Garden OA (2019) Phenotypic characterisation of regulatory T cells in dogs reveals signature transcripts conserved in humans and mice. Sci Rep 9:13478. https://doi.org/10.1038/s41598-019-50065-8
Jordan JT, Sun W, Hussain SF, DeAngulo G, Prabhu SS, Heimberger AB (2008) Preferential migration of regulatory T cells mediated by glioma-secreted chemokines can be blocked with chemotherapy. Cancer Immunol Immunother 57:123–131. https://doi.org/10.1007/s00262-007-0336-x
Bentley RT, Ahmed AU, Yanke AB, Cohen-Gadol AA, Dey M (2017) Dogs are man’s best friend: in sickness and in health. Neuro Oncol 19:312–322. https://doi.org/10.1093/neuonc/now109
Boudreau CE, Najem H, Ott M, Horbinski C, Fang D, DeRay CM, Levine JM, Curran MA, Heimberger AB (2021) Intratumoral delivery of STING Agonist results in clinical responses in canine glioblastoma. Clin Cancer Res 27:5528–5535. https://doi.org/10.1158/1078-0432.CCR-21-1914
Dickinson PJ, LeCouteur RA, Higgins RJ, Bringas JR, Larson RF, Yamashita Y, Krauze MT, Forsayeth J, Noble CO, Drummond DC, Kirpotin DB, Park JW, Berger MS, Bankiewicz KS (2010) Canine spontaneous glioma: a translational model system for convection-enhanced delivery. Neuro Oncol 12:928–940. https://doi.org/10.1093/neuonc/noq046
Blackmon AL, Pinter-Brown L (2020) Spotlight on Mogamulizumab-Kpkc for Use in adults with relapsed or refractory mycosis fungoides or Sezary syndrome: efficacy, safety, and patient selection. Drug Des Devel Ther 14:3747–3754. https://doi.org/10.2147/DDDT.S185896
Rodriguez-Perea AL, Arcia ED, Rueda CM, Velilla PA (2016) Phenotypical characterization of regulatory T cells in humans and rodents. Clin Exp Immunol 185:281–291. https://doi.org/10.1111/cei.12804
Li C, Jiang P, Wei S, Xu X, Wang J (2020) Regulatory T cells in tumor microenvironment: new mechanisms, potential therapeutic strategies and future prospects. Mol Cancer 19:116. https://doi.org/10.1186/s12943-020-01234-1
Sharma P, Allison JP (2020) Dissecting the mechanisms of immune checkpoint therapy. Nat Rev Immunol 20:75–76. https://doi.org/10.1038/s41577-020-0275-8
Delgoffe GM, Woo SR, Turnis ME, Gravano DM, Guy C, Overacre AE, Bettini ML, Vogel P, Finkelstein D, Bonnevier J, Workman CJ, Vignali DA (2013) Stability and function of regulatory T cells is maintained by a neuropilin-1-semaphorin-4a axis. Nature 501:252–256. https://doi.org/10.1038/nature12428
Teng MW, Ngiow SF, von Scheidt B, McLaughlin N, Sparwasser T, Smyth MJ (2010) Conditional regulatory T-cell depletion releases adaptive immunity preventing carcinogenesis and suppressing established tumor growth. Cancer Res 70:7800–7809. https://doi.org/10.1158/0008-5472.CAN-10-1681
Liu S, Zhang C, Wang B, Zhang H, Qin G, Li C, Cao L, Gao Q, Ping Y, Zhang K, Lian J, Zhao Q, Wang D, Zhang Z, Zhao X, Yang L, Huang L, Yang B, Zhang Y (2021) Regulatory T cells promote glioma cell stemness through TGF-beta-NF-kappaB-IL6-STAT3 signaling. Cancer Immunol Immunother 70:2601–2616. https://doi.org/10.1007/s00262-021-02872-0
Fecci PE, Sweeney AE, Grossi PM, Nair SK, Learn CA, Mitchell DA, Cui X, Cummings TJ, Bigner DD, Gilboa E, Sampson JH (2006) Systemic anti-CD25 monoclonal antibody administration safely enhances immunity in murine glioma without eliminating regulatory T cells. Clin Cancer Res 12:4294–4305. https://doi.org/10.1158/1078-0432.CCR-06-0053
Fecci PE, Ochiai H, Mitchell DA, Grossi PM, Sweeney AE, Archer GE, Cummings T, Allison JP, Bigner DD, Sampson JH (2007) Systemic CTLA-4 blockade ameliorates glioma-induced changes to the CD4 + T cell compartment without affecting regulatory T-cell function. Clin Cancer Res 13:2158–2167. https://doi.org/10.1158/1078-0432.CCR-06-2070
Jacobs JF, Idema AJ, Bol KF, Grotenhuis JA, de Vries IJ, Wesseling P, Adema GJ (2010) Prognostic significance and mechanism of Treg infiltration in human brain tumors. J Neuroimmunol 225:195–199. https://doi.org/10.1016/j.jneuroim.2010.05.020
Kohli K, Pillarisetty VG, Kim TS (2022) Key chemokines direct migration of immune cells in solid tumors. Cancer Gene Ther 29:10–21. https://doi.org/10.1038/s41417-021-00303-x
Cinier J, Hubert M, Besson L, Di Roio A, Rodriguez C, Lombardi V, Caux C, Menetrier-Caux C (2021) Recruitment and expansion of Tregs cells in the tumor environment-how to target them? Cancers (Basel) 13. https://doi.org/10.3390/cancers13081850
Acknowledgement
We want to thank all the companion dogs who participated in this study and their owners (Marguerite F Knipe, Christine Toedebusch, Amanda Dang, and Abrah Wymore) for donating blood samples to this work. The authors thank Katarzyna C. Pituch for technical advice on experimental design and statistical analysis. This research was supported by Dr. Terrell A. Holliday Grant Award by the American College of Veterinary Internal Medicine (ACVIM) (W.K.P) and Matching Fund Award by Center for Companion Animal Health, School of Veterinary Medicine, University of California, Davis (W.K.P); the UC Davis Paul Calabresi Career Development Award for Clinical Oncology as funded by the National Cancer Institute/National Institutes of Health through grant #2K12CA138464-11 (C.M.T.) and the Paul C. and Borghild T. Petersen Brain Tumor Foundation (W.K.P, R.G.T, P.J.D, and C.M.T). This project was also supported by the UC Davis Flow Cytometry Shared Resource Laboratory with funding from NCI P30 CA093373 (Comprehensive Cancer Center) and S10 OD018223– Beckman Coulter “Astrios” cell sorter and with technical assistance from Bridget McLaughlin and Jonathan Van Dyke.
Funding
Dr. Terrell A. Holliday Grant Award by the American College of Veterinary Internal Medicine (ACVIM) (W.K.P) and Matching Fund Award by Center for Companion Animal Health, School of Veterinary Medicine, University of California, Davis (W.K.P). This research was supported by the UC Davis Paul Calabresi Career Development Award for Clinical Oncology as funded by the National Cancer Institute/National Institutes of Health through grant #2K12CA138464-11 (C.M.T.) and the Paul C. and Borghild T. Petersen Brain Tumor Foundation (W.K.P, R.G.T, P.J.D, and C.M.T).
Author information
Authors and Affiliations
Contributions
All authors have significantly contributed to this manuscript. Conceptualization, W.K.P and C.M.T.; methodology, W.K.P and C.M.T.; investigation, W.K.P; J. M; R.G.T; B. M; K. D. W; M. B; M. S. L; P. J D; B. K. S; K. M. V; K. C. P; C. L and C.M.T.; writing - original draft preparation, W.K. P, C.M.T and P. J. D; writing - review and editing W.K.P; J. M; R.G.T; BE. M; K. D. W; M. E. B; M. S. L; P. J D; B. K. S; K. M. V; K; C. L and C.M.T, funding acquisition, W.K.P and C.M.T. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Panek, W.K., Toedebusch, R.G., Mclaughlin, B.E. et al. The CCL2-CCR4 axis promotes Regulatory T cell trafficking to canine glioma tissues. J Neurooncol 169, 647–658 (2024). https://doi.org/10.1007/s11060-024-04766-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-024-04766-4