Abstract
Purpose
To better define the role of surgery, we investigated survival and functional outcomes in patients with multiple brain metastases.
Methods
Pertinent clinical and radiological data of 131 consecutive patients (156 surgeries) were analyzed retrospectively.
Results
Surgical indications included mass effect (84.6%) and need for tissue acquisition (44.9%, for molecularly informed treatment: 10 patients). Major (i.e. CTCAE grade 3–5) neurological, surgical and medical complication were observed in 6 (3.8%), 12 (7.7%), and 12 (7.7%) surgical cases. Median preoperative and discharge KPS were 80% (IQF: 60–90%). Median overall survival (mOS) was 7.4 months. However, estimated 1 and 2 year overall survival rates were 35.6% and 25.1%, respectively. Survival was dismal (i.e. mOS ≤ 2.5 months) in patients who had no postoperative radio- and systemic therapy, or who incurred major complications. Multivariate analysis with all parameters significantly correlated with survival as univariate parameters revealed female sex, oligometastases, no major new/worsened neurological deficits, and postoperative radio- and systemic therapy as independent positive prognostic parameters. Univariate positive prognostic parameters also included histology (best survival in breast cancer patients) and less than median (0.28 cm3) residual tumor load.
Conclusions
Surgery is a reasonable therapeutic option in many patients with multiple brain metastases. Operations should primarily aim at reducing mass effect thereby preserving the patients’ functional health status which will allow for further local (radiation) and systemic therapy. Surgery for the acquisition of metastatic tissue (more recently for molecularly informed treatment) is another important surgical indication. Cytoreductive surgery may also carry a survival benefit by itself.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Given the poor survival outlook and systemic affection of the CNS, the role of surgery in cases with multiple brain metastases is controversial [1,2,3,4]. The literature contains few pertinent data collected against the background of the more recent developments in medical oncology [3, 5, 6]. Generally, patients with (multiple) brain metastases are considered for surgery for the following reasons: reduction of mass effect (i.e. treatment or prevention of neurological symptoms), acquiring tissue for histological and molecular genetic analysis, and possibly cytoreduction [4, 7,8,9]. Beyond this, certain histologies, such as renal carcinoma or malignant melanoma are not particularly radiation-sensitive which may favor surgical therapy over irradiation in such cases [7, 10].
Large space-occupying lesions lead to neurological deterioration which may prevent otherwise necessary oncological treatment both for CNS as well as systemic disease. In the posterior fossa, such lesions may be life-threatening due to an imminent risk of occlusive hydrocephalus and brainstem compression. Reduction of the mass effect of brain metastases may therefore be vital to keep and/or render patients oncologically “treatable” [6, 11].
The role of CNS tumor load and therefore cytoreduction has also attracted some attention [12,13,14]. More recently, the concept of oligometastatic disease has been introduced which establishes an intermediate category between (at least for some time) presumably localized disease i.e. cases with a single metastasis and patients with multiple metastases believed to suffer from a disseminated affection of the CNS [15, 16]. Oligometastatic brain disease is often defined as suffering from two to four brain metastases [15, 17, 18]. Patients with oligometastases may benefit from therapeutic strategies aiming at local rather than systemic CNS tumor control even though several “loci” need to be addressed [4, 9, 15].
Not rarely, brain metastases are the first manifestation of the primary disease. In some cases, staging studies fail to identify a primary tumor or the presumed primary tumor is not readily accessible so that brain metastases surgery is needed for diagnostic purposes. Some tumors, such as breast cancer or malignant melanoma, show genetic discrepancies between the metastatic disease and primary tumor [19, 20]. Amid growing targeted-therapy options, acquisition of metastatic tissue may help guiding (medical) therapy (“molecularly informed treatment”) [21, 22].
The aim of the present study was to investigate the role of surgery for patients with multiple brain metastases in a large patient cohort treated in view of recent advances in medical and radio-oncology. The current literature contains only limited data on complications and functional outcomes following surgery for brain metastases [5, 6]. Can we identify patients who presumably derive a survival benefit? What is the role of surgical cytoreduction (if there is any)?
Patients & methods
Patients
We conducted a retrospective analysis of all consecutive patients with multiple brain metastases undergoing surgery for at least one of the tumors from January 2015 to July 2021 in our department. Cases with stereotactic biopsies only were excluded. The study was approved by the responsible institutional review board for human research and ethics committee.
All cases were discussed in the interdisciplinary neuro-oncology tumor board and additionally in disease-specific tumor boards as required. If reasonably possible, a locally aggressive approach was pursued, i.e. an attempt was made to remove all metastatic disease either by means of surgery or by combing surgery and radiosurgery [3, 23, 24].
Surgical indications
Patients were considered as candidates for surgery when they presented with a large space-occupying tumor either already causing or believed to pose a significant risk for developing neurological deficits as long as a valid postoperative oncological therapeutic concept was available. This specifically included cases with metastases in the posterior fossa and (imminent) hydrocephalus and brainstem compression. Surgery was also performed in order to obtain tumor tissue whenever a histological diagnosis was needed and/or when primary tumor tissue was not readily accessible for diagnosis confirmation, and in some cases which required molecular studies for optimal therapeutic management. If reasonably possible, a locally aggressive approach was pursued, i.e. an attempt was made to remove all metastatic disease either by means of surgery or by combing surgery and radiosurgery, assuming that this would result in superior survival [3, 23, 24].
All cases were discussed in the interdisciplinary neuro-oncology tumor board and additionally in disease-specific tumor boards as required. Neuronavigation and in cases with a presumed eloquent or semi-eloquent location [25] intraoperative neuromonitoring/awake craniotomy were routinely used.
Clinical data and follow-up
Pertinent clinical and radiological data were retrieved through a chart review and– if necessary - telephone interviews. All relevant pre- and postoperative oncological treatments as well as cause of death (CNS-disease related or systemic) were documented. Complications were documented using the CTCAE classification framework (Common Terminology Criteria for Adverse Events v5.0; https://ctep.cancer.gov) and recorded in three categories: surgical, medical, and persisting (≥ 30 days following the index surgery) neurological deficits [11].
Imaging data and volumetric analysis
Preoperative MRI investigations were available for 128 patients (97.7%). 81/131 (61.8%) patients received a postoperative MRI within 72 h of surgery, whereas 45/131 (34.4%) received a CT and 5/131 (3.8%) no early postoperative imaging study. Volumetric analysis was conducted using pre- and postoperative MRI studies and a commercially available software (iplanNet, Brainlab AG, Munich, Germany). We recorded volume of the index metastasis/-es (128 [97.7%] patients), preoperative total tumor load (excluding 4 cases with meningiosis carcinomatosa: 124 [94.7%] patients), and residual total tumor load (i.e. volume of all metastatic disease after removal of the index metastases, excluding the meningiosis cases: 78 [59.5%] patients). All surgeries were also assessed whether they addressed tumors in an eloquent location based on the preoperative imaging studies using the paradigm developed by Sawaya [25].
Statistical analysis
Commercially available IBM SPSS Statistics for Windows software (Version 25.0, IBM Corp., Armonk, NY) was used for statistical analysis. Kaplan-Meier estimates and log-rank rests were used to study overall survival. Cox and binary logistic regression analyses were performed for multivariate analysis.
Results
Patient cohort & tumor characteristics
The series comprised 131 patients (156 surgeries). Median age was 62.0 (IQF 25–75%: 54.0–70.0, range: 32–85) years. Forty-six cases (34.4%) were diagnosed with two metastases. Three and four metastases were present in 22 (14.1%) and 18 (11.5%) cases, respectively (median: 3, IQR 25–75%: 2–6, range: 2–34), i.e. 82 (62.6%) patients had oligometastatic (2–4 tumors) disease [15, 17, 18]. Primary tumors included lung cancer in 67 (51.1%; NSCLC: 51, SCLC: 14, NET/lung neuroendocrine tumor: 2), breast cancer in 26 (19.8%; ER-/PR-/HER2-: 5, ER+/PR+/HER2-: 7, HER2+: 7, N/A: 7), melanoma in 7 (5.3%), colo-rectal carcinoma in 7 (5.3%), and renal cell carcinoma in 4 (3.1%) patients. Five cases (3.8%) were diagnosed with CUP (cancer of unknown primary) syndrome.
Molecular studies of potential targets for therapeutic interventions were performed in 38 patients (breast: 23, lung: 9, melanoma: 2, other: 4). In 3/17 assessable breast cancer cases ER/PR/HER2 status differed between primary tumor and the brain metastasis revealing a new clinically actionable target (i.e. HER2+) [26]. Overall, 14 breast cancer metastases were HER2+. Two NSCLC metastasis tested positive for PD-L-1 which allowed for treatment with pembrolizumab [27].
107/131 (81.2%) patients were followed until death, and median follow-up for patients alive at the time of analysis was 30.3 months (IQR 25–75%: 21.8–43.3). Further characteristics of the cohort can be found in Tables 1 and 2.
Surgical treatment & adjuvant therapies
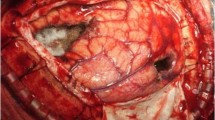
Twenty-three patients underwent two and two patients had three operations. One patient underwent a re-resection of residual tumor. Patients often had more than one indication to undergo surgery. Surgical indications included mass effect (posterior fossa: 62, supratentorial disease: 68, both: 2), no known primary cancer (50), history of multiple cancers (12), known radioresistant primary tumor (7), and tumor progress after radiosurgery/-therapy (3). In 10 patients with a known primary tumor, tissue acquisition was nevertheless requested by the treating oncologist specifically for additional molecular pathological studies. Examples of surgical indications are shown in Fig. 1. Neuromonitoring and/or awake craniotomy were used in 38 surgeries. In 40 cases (30.5%) all metastatic deposits were addressed by local treatments (i.e. surgery: 29 [22.1%], surgery & radiosurgery: 11 [8.4%]).
Examples of surgical indications (T1 weighted & contrast-enhanced MR images) a 51-year-old female patient with multiple brain metastases in the posterior fossa (arrows) and known esophageal cancer. Surgery was performed primarily in order to avoid occlusive hydrocephalus, i.e. only the large left mass consisting of several coalescing metastases was removed. The patient survived for 34 months following the operation. b 35-year-old female with breast cancer (ER+, PR+, HER2+), hepatic metastases diagnosed 41 months earlier and four brain metastases (arrows). She presented with headaches and a visual field cut. The treating oncologist requested tissue for molecular analyses (“molecularly informed treatment”). We felt that this case might benefit from aggressive local treatment of all CNS cancer manifestations. Hence, the patient had two surgeries for removal (1) of the left cerebellar and temporo-parietal metastases and (2) the right fronto-basal tumor. All tumor were HER+, but ER-/PR-. The small right cerebellar metastasis was treated with radiosurgery. We favored surgery for the frontal and the temporo-parietal lesions in order to save radiosurgery as an option for tumor recurrence. Indeed, despite adjuvant radiotherapy further radiosurgeries and even one additional surgery for a left cerebellar recurrence were required later on for CNS disease control. The patient was alive and well at her last follow-up 30 months after the initial operations. C 58-year-old male patient presenting with multiple brain metastases (arrows; additional small tumors in the left temporal and both occipital lobes), mild aphasia and no known primary tumor. Initial staging investigations suggested lung cancer as the primary tumor. However, a bronchoscopy failed to establish a tissue diagnosis. Awake surgery was performed for removal of the left frontal lesion in order to relieve the mass effect and its consecutive neurological symptoms (aphasia) and to obtain a tissue diagnosis. The patient improved and was neurologically intact after the surgery. Pathological evaluation of the CNS metastasis revealed carcinoma with neuroendocrine differentiation well in line with the presumptive diagnosis of primary lung cancer. d 66-year-old male patient diagnosed with malignant melanoma 10 years earlier and presenting with moderate paresis of the right hand > arm. The tumors (arrows) were removed through two separate craniotomies with the help of intraoperative MEP neuromonitoring. Surgery was favored over primary radiotherapy/-surgery because of the presumed resistance of malignant melanoma CNS metastases to radiotherapy and in an attempt to preserve the patient’s neurological status though removal of the lesion in the right precentral gyrus. The patient’s neurodeficit indeed improved slightly after surgery
Postoperative radiotherapy was administered in 94/130 (72.3%) cases and systemic therapy in 76/127 (59.8%) patients. Thirty-one (24.4%) of 127 cases had no adjuvant treatments.
Complications & functional outcomes
Major (i.e. CTCAE grade 3–5) neurological, surgical and medical complication were observed in 6 (3.8%), 12 (7.7%), and 12 (7.7%) surgical cases. Interestingly, the neurodeficit rates did not vary significantly with the eloquence of the tumor location (eloquent: 0/19 [0%], semi-eloquent: 1/24 [4.2%], non-eloquent: 5/113 [4.4%], p = NS).
Median discharge KPS was 80% (IQF: 60–90%, range 0-100%). Following surgery, the KPS improved in 38/131 (29.0%). A worsening of the KPS was seen in 29/131 (22.1%; ≥ 20: 16 [12.2%]). Univariate predictors of a good functional outcome (KPS 90–100%) included younger age, female sex, better preoperative KPS, (semi)-eloquent location of the index metastasis/-es, histology (best functional outcomes in breast cancer cases), and no major surgical or medical complication (Table 1). A multivariate analysis with all variables which correlated significantly with better KPS outcomes in the univarate analysis showed statistically significant associations for a higher preoperative KPS, no major postoperative complication, and a (semi)-eloquent tumor location (Table 3).
Of note, patients with a better discharge KPS had significantly more often systemic treatments (KPS 90–100: 47/53 [88.7%] vs. KPS ≤ 80: 29/74 [39.2%], p < 0.001) and/or radiotherapy (KPS 90–100: 51/54 [94.4%] vs. KPS ≤ 80: 43/76 [56.6%], p < 0.001) after their operation. 31 cases (23.8%, unknown: 1) had no postoperative systemic or radiotherapy. 22/31 (71.0%) of these cases had a KPS of 60% or worse, i.e. an adverse functional health status was the major reason for not undergoing postoperative treatment. None of the cases with a new/worsened major neurodeficit had radiotherapy (p < 0.001) or adjuvant systemic treatment (p = 0.009). Surgical and medical complications also correlated inversely with postoperative treatment; however, these findings were statistically significant only for medical complications and systemic therapies.
Patient survival
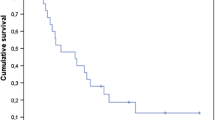
Median overall survival (mOS) was 7.4 months (95% CI: 5.4–9.5). Estimated 1 year overall survival rate was 35.6%, and the 2 year rate was 25.1%. 30-day-mortality was 10.7% (14/131) including 10 (7.6%) patients who died from complications of the primary disease unrelated to the index surgery. Cause of death was available for 106 (80.9%) patients. 25/106 (23.6%) died due to CNS disease.
Positive univariate predictors of overall survival included younger age (≤ 62 years), female sex, higher pre- (day 1 before surgery) and postoperative KPS (i.e. at discharge, assessed at 5 days (median, IQR 25–75%: 6.0–14.0) after surgery) and oligometastatic (2–4 tumors) disease. Patients with oligometastases had significantly higher pre- and postoperative KPS, and significantly lower residual tumors volumes (Supplementary Table 1). Survival also varied strongly with primary tumor histology, e.g. mOS in patients with breast cancer was 67.2 months, and the estimated 3-year overall survival rate was 55.6%. The presence of extracerebral metastases did not impact on OS. Survival was dismal (i.e. mOS ≤ 2.5 months) in patients who had no postoperative radio- and systemic therapy, or who incurred major complications (see Table 2). We investigated the role of postoperative treatment separately in patients with lung cancer, i.e. the largest subgroup defined by their primary cancer. Patients who received postoperative radio- and systemic treatment had a significant better overall survival: (mOS, surgery only: 0.9 months (95% CI 0.2–1.6) vs. surgery and postoperative monotherapy: 3.2 months (2.4-4.0) vs. surgery and radio- as well as systemic therapy: 13.6 months (8.0-19.2); p < 0.001)).
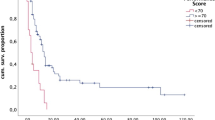
Preoperative total tumor load did not influence OS. Likewise, the volume of the index metastases was not prognostic. Aggressive local therapy (surgery and/or radiosurgery) for all brain metastases did not carry a survival benefit in our cohort. However, extent of resection was prognostic. Using median residual tumor volume (0.28 cm3) as the cut-off, patients with less residual tumor had a significantly better mOS (10.7 months, cf. 5.8 months for cases with > 0.28 cm3 residual tumor; p = 0.012; Table 2; Fig. 2).
Impact of selected prognostic parameters on overall survival (Kaplan Meier estimates) a Extent of resection. A survival benefit is associated with resections resulting in ≤ 0.28 cm3 residual tumor (≈ corresponding to a spherical lesion with a 0.8 cm diameter) b Extent of CNS disease. Survival is better in patients with oligometastases (2–4) vs. polymetastases (> 4). c Primary tumor histology. The relatively best survival was seen in patients with breast cancer. d New/worsend major (CTCAE grade 3–5) neurodeficit. Neurological worsening is associated with a dismal prognosis. e Postoperative radiotherapy & f Postoperative systemic therapy. Overall survival of cases not undergoing postoperative radiotherapy and/or systemic treatment is very poor reflecting the importance of local CNS as well as systemic disease control but also the role of maintaining/improving the functional status in order to enable the patient to undergo postoperative treatments
Multivariate analysis with all parameters significantly correlated with survival as univariate parameters revealed female sex, oligometastatic (2–4 tumors) disease, no major new/worsened neurodeficit, and postoperative radio- and systemic therapy as independent positive prognostic parameters in our cohort (Table 4).
Discussion
For the present paper we have reviewed our 2015–2021 institutional experience with the surgical treatment of patients with multiple brain metastases. Similar to what has been reported in the literature [28,29,30], survival in our cohort was limited (median overall survival: 7.4 months), however, varied greatly between patients. Comparing the results reported in surgical series with the radiotherapy [31, 32] and radiosurgery literature [33] is very difficult due to the inherent heavy patient selection bias. A very sizable percentage of the patients with multiple brain metastases will survive beyond 1–2 years. Of note, most patients (including our own) described in surgical multiple brain metastases series harbor only 2 to 4 metastases, i.e. suffer from oligometastases [15, 17, 18]. Our data suggest a prominent role for the extent of the CNS disease (assessed by number of brain metastases) as a prognostic factor and thereby support the concept of CNS oligometastases.
Operating patients in order to obtain tissue (diagnoses) appears to play a most important role in the management of cases with multiple brain metastases. For instance, surgical indications in our cohort included no known primary cancer in as many as 38.2% and a history of multiple cancers in 9.2%. Surgery was performed in 7.6% of our cases in order to access tissue for biomarker/target analysis. This is a relatively new indication and may well be performed increasingly more often in the future [34]. Patients with breast cancer from this series had a much better prognosis than the remainder of our cohort, which would also explain in part superior survival seen in females. Explanations of this finding certainly include selection bias and the very limited number of cases analyzed. The relative percentage of HER2+ (7/19 [36.8%]) but also of prognostically unfavorable triple-negative (ER-/PR-/HER2-) cases was similar to a recently reported large cohort of breast cancer patients with CNS metastases [35]. Interestingly, Sperduto et al. [3] described overall improving survival in patients with brain metastases in general, but in particular in breast cancer cases. Contradictory findings have also been reported [8].
The strong impact of postoperative radiotherapy and systemic treatment on survival highlights the paramount importance of both local (CNS) as well as systemic disease control. Postoperative irradiation is an integral part of most therapeutic concepts aiming at CNS disease control [36, 37]. Our data indicate that the primary role of surgery in patients with multiple brain metastases is to enable them to undergo further oncological treatment. Most agents used for systemic therapies clinically do not work for brain metastases. Hence, the major prognostic role of systemic tumor therapy likely reflects the importance of treating the systemic cancer once the CNS disease is controlled. Only approximately one quarter of our cases eventually died from CNS disease. This figure suggests that current local therapeutic concepts for multiple brain metastases work reasonably well, so that future advances in systemic oncological therapy may hopefully translate into significantly improved survival in these patients.
Complications and an adverse functional health status very often preclude postoperative treatments which at least in part explains their negative prognostic impact. In our cohort, new persistent CTCAE grade 3–5 neurological deficits were seen in 3.8%, the corresponding figures for surgical and medical complications were 7.7% and 7.7%, respectively. Most of our patients retained their preoperative KPS. An improved KPS was seen in 38/131 (29.0%), but also KPS worsening (e.g. 22.1% KPS drop ≥ 20) in a sizable number of cases. Similar figures (including the sizable proportion of cases with functional improvement following surgery) have also been published by others [3, 5,6,7, 9, 28, 38].
Surgical strategies therefore have to pay much attention to complication avoidance [7, 11]. (Piecemeal) surgical removal and opening of the ventricular system have been discussed as potential risk factors for secondary leptomeningeal dissemination (LMD) after surgery [39, 40]. We routinely use techniques and adjuncts such as intraoperative monitoring and awake craniotomies (Fig. 1) aiming at preserving neurological function similar as in operations for intrinsic tumors. Interestingly, eloquence of the tumor location did not negatively impact on neurodeficit rates in this series. Functional outcomes were even better in patients with surgery for (semi)-eloquent tumors. Besides selection bias, this may in part reflect the role of surgery in preserving or even improving function. At least these data suggest that an eloquent location of brain metastases should not deter from surgical treatment.
The most controversial indication for surgery in cases with multiple brain metastases may well be surgical cytoreduction beyond relieving mass effect [12,13,14, 41]. Less than median residual metastatic disease volume (= 0.28 cm3) correlated significantly with better survival in the present series. However, this effect was lost in the multivariate analysis. A 0.28 cm3 tumor residual would correspond to a spheric lesion with a diameter of 0.8 cm. This figure is very similar to the 1 cm cut-off used to distinguish between measurable and non-measurable disease in the RANO criteria [42]. Hence, our data may indicate that surgical cytoreduction aiming at removing all “measurable” disease may carry a survival benefit. It should be noted, however, that our data somewhat contradictorily did not confirm that a policy of addressing all brain metastases with surgery and/or radiosurgery carries a survival benefit [3, 23, 24, 43].
Our study has significant limitations. Albeit studying consecutive patients, data acquisition and analysis was performed retrospectively. Volumetric analyses were not available for all patients. Many patients received their postoperative treatment in outside institutions. Finally, our analysis cannot account for the very significant selection bias that not all patients with multiple brain metastases are referred for surgical treatment or at least a neurosurgical opinion.
Nevertheless, taken together, our investigation provides robust data to suggest that patients with multiple brain metastases can benefit from surgery most likely because surgical tumor removal reduces mass effect and thereby often helps to maintain or even improve a patient’ functional status which creates the necessary time for effective radio- and medical oncological therapy [6, 41].
Conclusion
Surgery is a reasonable therapeutic option in many patients with multiple brain metastases despite their overall poor prognosis. Diagnostic surgery for the acquisition of metastatic tissue (more recently also to help with the identification of potentially “drugable” targets) is an important surgical indication. Operations should primarily aim at preserving or even improving the patients’ functional health status in order to enable them to undergo further local (usually radiation) and systemic therapy. The surgical goal is a maximum safe cytoreduction (which may carry a survival benefit by itself) and therefore may require the routine use of neuromonitoring and/or awake craniotomy.
Data Availability
No datasets were generated or analysed during the current study.
References
Stelzer KJ (2013) Epidemiology and prognosis of brain metastases. Surg Neurol Int 4:S192–202. https://doi.org/10.4103/2152-7806.111296
Gaspar LE, Mehta MP, Patchell RA, Burri SH, Robinson PD, Morris RE, Ammirati M, Andrews DW, Asher AL, Cobbs CS, Kondziolka D, Linskey ME, Loeffler JS, McDermott M, Mikkelsen T, Olson JJ, Paleologos NA, Ryken TC, Kalkanis SN (2010) The role of whole brain radiation therapy in the management of newly diagnosed brain metastases: a systematic review and evidence-based clinical practice guideline. J Neurooncol 96:17–32. https://doi.org/10.1007/s11060-009-0060-9
Salvati M, Tropeano MP, Maiola V, Lavalle L, Brogna C, Colonnese C, Frati A, D’Elia A (2018) Multiple brain metastases: a surgical series and neurosurgical perspective. Neurol Sci off J Ital Neurol Soc Ital Soc Clin Neurophysiol 39:671–677. https://doi.org/10.1007/s10072-017-3220-2
Hatiboglu MA, Akdur K, Sawaya R (2020) Neurosurgical management of patients with brain metastasis. Neurosurg Rev 43:483–495. https://doi.org/10.1007/s10143-018-1013-6
Jünger ST, Reinecke D, Meissner A-K, Goldbrunner R, Grau S (2022) Resection of symptomatic non-small cell lung cancer brain metastasis in the setting of multiple brain metastases. J Neurosurg 136:1576–1582. https://doi.org/10.3171/2021.7.JNS211172
Schödel P, Jünger ST, Wittersheim M, Reinhardt HC, Schmidt N-O, Goldbrunner R, Proescholdt M, Grau S (2020) Surgical resection of symptomatic brain metastases improves the clinical status and facilitates further treatment. Cancer Med 9:7503–7510. https://doi.org/10.1002/cam4.3402
Hatiboglu MA, Wildrick DM, Sawaya R (2013) The role of surgical resection in patients with brain metastases. Ecancermedicalscience 7:308. https://doi.org/10.3332/ecancer.2013.308
Hardesty DA, Nakaji P (2016) The current and future treatment of Brain metastases. Front Surg 3:30. https://doi.org/10.3389/fsurg.2016.00030
Proescholdt MA, Schödel P, Doenitz C, Pukrop T, Höhne J, Schmidt NO, Schebesch K-M (2021) The management of Brain metastases-systematic review of neurosurgical aspects. Cancers (Basel) 13. https://doi.org/10.3390/cancers13071616
Pollock BE, Brown PD, Foote RL, Stafford SL, Schomberg PJ (2003) Properly selected patients with multiple brain metastases may benefit from aggressive treatment of their intracranial disease. J Neurooncol 61:73–80. https://doi.org/10.1023/a:1021262218151
Ersoy TF, Mokhtari N, Brainman D, Berger B, Salay A, Schütt P, Weissinger F, Grote A, Simon M (2021) Surgical Treatment of Cerebellar metastases: Survival benefits, complications and timing issues. Cancers (Basel) 13. https://doi.org/10.3390/cancers13215263
Aftahy AK, Barz M, Lange N, Baumgart L, Thunstedt C, Eller MA, Wiestler B, Bernhardt D, Combs SE, Jost PJ, Delbridge C, Liesche-Starnecker F, Meyer B, Gempt J (2022) The impact of postoperative tumor burden on patients with brain metastases. Front Oncol 12:869764. https://doi.org/10.3389/fonc.2022.869764
Yang K, Gutiérrez-Valencia E, Landry AP, Kalyvas A, Millesi M, Leite M, Jablonska PA, Weiss J, Millar B-A, Conrad T, Laperriere N, Bernstein M, Zadeh G, Shultz D, Kongkham PN (2022) Multiplicity does not significantly affect outcomes in brain metastasis patients treated with surgery. Neuro-oncology Adv 4:vdac022. https://doi.org/10.1093/noajnl/vdac022
Baumgart L, Aftahy AK, Anetsberger A, Thunstedt D, Wiestler B, Bernhardt D, Combs SE, Meyer B, Meyer HS, Gempt J (2023) Brain metastases in the elderly - impact of residual tumor volume on overall survival. Front Oncol 13:1149628. https://doi.org/10.3389/fonc.2023.1149628
Ramos A, Giantini-Larsen A, Pannullo SC, Brandmaier A, Knisely J, Magge R, Wilcox JA, Pavlick AC, Ma B, Pisapia D, Ashamalla H, Ramakrishna R (2022) A multidisciplinary management algorithm for brain metastases. Neuro-oncology Adv 4:vdac176. https://doi.org/10.1093/noajnl/vdac176
Mazzola R, Corradini S, Gregucci F, Figlia V, Fiorentino A, Alongi F (2019) Role of Radiosurgery/Stereotactic Radiotherapy in Oligometastatic Disease: Brain oligometastases. Front Oncol 9:206. https://doi.org/10.3389/fonc.2019.00206
Grossenbacher B, Lareida A, Moors S, Roth P, Kulcsar Z, Regli L, Le Rhun E, Weller M, Wolpert F (2023) Prognostic assessment in patients operated for brain metastasis from systemic tumors. Cancer Med 12:12316–12324. https://doi.org/10.1002/cam4.5928
Nieder C, Hintz M, Popp I, Bilger A, Grosu AL (2020) Long-term survival results after treatment for oligometastatic brain disease. Rep Pract Oncol Radiother J Gt Cancer Cent Pozn Pol Soc Radiat Oncol 25:307–311. https://doi.org/10.1016/j.rpor.2020.03.001
Brastianos PK, Carter SL, Santagata S, Cahill DP, Taylor-Weiner A, Jones RT, Van Allen EM, Lawrence MS, Horowitz PM, Cibulskis K, Ligon KL, Tabernero J, Seoane J, Martinez-Saez E, Curry WT, Dunn IF, Paek SH, Park S-H, McKenna A, Chevalier A, Rosenberg M, Barker FG 2nd, Gill CM, Van Hummelen P, Thorner AR, Johnson BE, Hoang MP, Choueiri TK, Signoretti S, Sougnez C, Rabin MS, Lin NU, Winer EP, Stemmer-Rachamimov A, Meyerson M, Garraway L, Gabriel S, Lander ES, Beroukhim R, Batchelor TT, Baselga J, Louis DN, Getz G, Hahn WC (2015) Genomic characterization of Brain metastases reveals branched evolution and potential therapeutic targets. Cancer Discov 5:1164–1177. https://doi.org/10.1158/2159-8290.CD-15-0369
Duchnowska R, Sperinde J, Chenna A, Huang W, Weidler JM, Winslow J, Haddad M, Paquet A, Lie Y, Trojanowski T, Mandat T, Kowalczyk A, Czartoryska-Arłukowicz B, Radecka B, Jarosz B, Staszkiewicz R, Kalinka-Warzocha E, Chudzik M, Biernat W, Jassem J (2015) Quantitative HER2 and p95HER2 levels in primary breast cancers and matched brain metastases. Neuro Oncol 17:1241–1249. https://doi.org/10.1093/neuonc/nov012
Le Scodan R, Massard C, Jouanneau L, Coussy F, Gutierrez M, Kirova Y, Lerebours F, Labib A, Mouret-Fourme E (2012) Brain metastases from breast cancer: proposition of new prognostic score including molecular subtypes and treatment. J Neurooncol 106:169–176. https://doi.org/10.1007/s11060-011-0654-x
Sperduto PW, Jiang W, Brown PD, Braunstein S, Sneed P, Wattson DA, Shih HA, Bangdiwala A, Shanley R, Lockney NA, Beal K, Lou E, Amatruda T, Sperduto WA, Kirkpatrick JP, Yeh N, Gaspar LE, Molitoris JK, Masucci L, Roberge D, Yu J, Chiang V, Mehta M (2017) The Prognostic Value of BRAF, C-KIT, and NRAS mutations in Melanoma patients with brain metastases. Int J Radiat Oncol Biol Phys 98:1069–1077. https://doi.org/10.1016/j.ijrobp.2017.03.030
Bindal RK, Sawaya R, Leavens ME, Lee JJ (1993) Surgical treatment of multiple brain metastases. J Neurosurg 79:210–216. https://doi.org/10.3171/jns.1993.79.2.0210
Iwadate Y, Namba H, Yamaura A (2000) Significance of surgical resection for the treatment of multiple brain metastases. Anticancer Res 20:573–577
Sawaya R, Hammoud M, Schoppa D, Hess KR, Wu SZ, Shi WM, Wildrick DM (1998) Neurosurgical outcomes in a modern series of 400 craniotomies for treatment of parenchymal tumors. Neurosurgery 42:1044–1046. https://doi.org/10.1097/00006123-199805000-00054
Lin NU, Murthy RK, Abramson V, Anders C, Bachelot T, Bedard PL, Borges V, Cameron D, Carey LA, Chien AJ, Curigliano G, DiGiovanna MP, Gelmon K, Hortobagyi G, Hurvitz SA, Krop I, Loi S, Loibl S, Mueller V, Oliveira M, Paplomata E, Pegram M, Slamon D, Zelnak A, Ramos J, Feng W, Winer E (2023) Tucatinib vs Placebo, both in Combination with Trastuzumab and Capecitabine, for previously treated ERBB2 (HER2)-Positive metastatic breast Cancer in patients with brain metastases: updated exploratory analysis of the HER2CLIMB Randomized Clinical Trial. JAMA Oncol 9:197–205. https://doi.org/10.1001/jamaoncol.2022.5610
Goldberg SB, Schalper KA, Gettinger SN, Mahajan A, Herbst RS, Chiang AC, Lilenbaum R, Wilson FH, Omay SB, Yu JB, Jilaveanu L, Tran T, Pavlik K, Rowen E, Gerrish H, Komlo A, Gupta R, Wyatt H, Ribeiro M, Kluger Y, Zhou G, Wei W, Chiang VL, Kluger HM (2020) Pembrolizumab for management of patients with NSCLC and brain metastases: long-term results and biomarker analysis from a non-randomised, open-label, phase 2 trial. Lancet Oncol 21:655–663. https://doi.org/10.1016/S1470-2045(20)30111-X
Proescholdt M, Jünger ST, Schödel P, Schebesch K-M, Doenitz C, Pukrop T, Höhne J, Schmidt N-O, Kocher M, Schulz H, Ruge M, König K, Goldbrunner R, Grau S (2021) Brain metastases in Elderly patients-the role of surgery in the context of systemic treatment. Brain Sci 11. https://doi.org/10.3390/brainsci11010123
Pojskic M, Bopp MHA, Schymalla M, Nimsky C, Carl B (2017) Retrospective study of 229 surgically treated patients with brain metastases: prognostic factors, outcome and comparison of recursive partitioning analysis and diagnosis-specific graded prognostic assessment. Surg Neurol Int 8:259. https://doi.org/10.4103/sni.sni_228_17
Sperduto PW, Mesko S, Li J, Cagney D, Aizer A, Lin NU, Nesbit E, Kruser TJ, Chan J, Braunstein S, Lee J, Kirkpatrick JP, Breen W, Brown PD, Shi D, Shih HA, Soliman H, Sahgal A, Shanley R, Sperduto WA, Lou E, Everett A, Boggs DH, Masucci L, Roberge D, Remick J, Plichta K, Buatti JM, Jain S, Gaspar LE, Wu C-C, Wang TJC, Bryant J, Chuong M, An Y, Chiang V, Nakano T, Aoyama H, Mehta MP (2020) Survival in patients with brain metastases: Summary Report on the updated diagnosis-specific graded Prognostic Assessment and Definition of the eligibility quotient. J Clin Oncol off J Am Soc Clin Oncol 38:3773–3784. https://doi.org/10.1200/JCO.20.01255
Karlsson AT, Hjermstad MJ, Aass N, Skovlund E, Kaasa S, Yri OE (2024) Overall survival after Radiotherapy for Brain metastases according to ECOG Status-A prospective study of 294 NSCLC patients. Cancers (Basel) 16. https://doi.org/10.3390/cancers16081486
Flores-Paco P, Vargas-Aliaga A, Guevara MG, Lopera I, Ruiz LR, López-Herrero M, Camús JA, López-González J, Inga-Saavedra E, Montero M, Barneto I, Gómez-España MA, Ruiz E, Ruza M, Armenta A, Palacios A, De La Haba-Rodríguez JR, Aranda E (2024) A new updated prognostic index for patients with brain metastases (BMs) treated with palliative whole brain radiotherapy (WBRT) in the era of precision oncology. METASNCore project. J Neurooncol 167:407–413. https://doi.org/10.1007/s11060-024-04618-1
Bodensohn R, Maier SH, Belka C, Minniti G, Niyazi M (2023) Stereotactic radiosurgery of multiple brain metastases: a review of treatment techniques. Cancers (Basel) 15. https://doi.org/10.3390/cancers15225404
Le Rhun E, Guckenberger M, Smits M, Dummer R, Bachelot T, Sahm F, Galldiks N, de Azambuja E, Berghoff AS, Metellus P, Peters S, Hong Y-K, Winkler F, Schadendorf D, van den Bent M, Seoane J, Stahel R, Minniti G, Wesseling P, Weller M, Preusser M (2021) EANO-ESMO clinical practice guidelines for diagnosis, treatment and follow-up of patients with brain metastasis from solid tumours. Ann Oncol off J Eur Soc Med Oncol 32:1332–1347. https://doi.org/10.1016/j.annonc.2021.07.016
Riecke K, Müller V, Neunhöffer T, Park-Simon T-W, Weide R, Polasik A, Schmidt M, Puppe J, Mundhenke C, Lübbe K, Hesse T, Thill M, Wuerstlein R, Denkert C, Decker T, Fehm T, Nekljudova V, Rey J, Loibl S, Laakmann E, Witzel I (2023) Long-term survival of breast cancer patients with brain metastases: subanalysis of the BMBC registry. ESMO open 8:101213. https://doi.org/10.1016/j.esmoop.2023.101213
Patchell RA, Tibbs PA, Walsh JW, Dempsey RJ, Maruyama Y, Kryscio RJ, Markesbery WR, Macdonald JS, Young B (1990) A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med 322:494–500. https://doi.org/10.1056/NEJM199002223220802
Vecht CJ, Haaxma-Reiche H, Noordijk EM, Padberg GW, Voormolen JH, Hoekstra FH, Tans JT, Lambooij N, Metsaars JA, Wattendorff AR (1993) Treatment of single brain metastasis: radiotherapy alone or combined with neurosurgery? Ann Neurol 33:583–590. https://doi.org/10.1002/ana.410330605
Schackert G, Steinmetz A, Meier U, Sobottka SB (2001) Surgical management of single and multiple brain metastases: results of a retrospective study. Onkologie 24:246–255. https://doi.org/10.1159/000055087
Tewarie IA, Jessurun CAC, Hulsbergen AFC, Smith TR, Mekary RA, Broekman MLD (2021) Leptomeningeal disease in neurosurgical brain metastases patients: a systematic review and meta-analysis. Neuro-oncology Adv 3:vdab162. https://doi.org/10.1093/noajnl/vdab162
DePaoli B, Gozal YM, Pater LE, Breneman JC, Warnick RE, Elson J, Struve TD (2019) Ventricular violation increases the risk of leptomeningeal disease in cavity-directed radiosurgery treated patients. J Radiat Oncol 8:23–29. https://doi.org/10.1007/s13566-018-0368-1
Hong N, Yoo H, Gwak HS, Shin SH, Lee SH (2013) Outcome of surgical resection of symptomatic cerebral lesions in non-small cell lung cancer patients with multiple brain metastases. Brain Tumor Res Treat 1:64–70. https://doi.org/10.14791/btrt.2013.1.2.64
Lin NU, Lee EQ, Aoyama H, Barani IJ, Barboriak DP, Baumert BG, Bendszus M, Brown PD, Camidge DR, Chang SM, Dancey J, de Vries EGE, Gaspar LE, Harris GJ, Hodi FS, Kalkanis SN, Linskey ME, Macdonald DR, Margolin K, Mehta MP, Schiff D, Soffietti R, Suh JH, van den Bent MJ, Vogelbaum MA, Wen PY (2015) Response assessment criteria for brain metastases: proposal from the RANO group. Lancet Oncol 16:e270–e278. https://doi.org/10.1016/S1470-2045(15)70057-4
Bschorer M, Ricklefs FL, Sauvigny T, Westphal M, Dührsen L (2023) Multiple craniotomies in a single surgery - the resection of scattered brain metastases. Neurosurg Rev 46:70. https://doi.org/10.1007/s10143-023-01976-8
Acknowledgements
We thank Dr. Attila Salay (radiation oncologist), Dr. Philipp Schütt (medical oncologist), Dr. Marianne Just (medical oncologist), and Prof. Oliver Micke (radiation oncologist) for providung us oncological follow-up information.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
Conceptualization: [Tunc F. Ersoy, Matthias Simon]; Methodology: [Tunc F. Ersoy, Matthias Simon], Formal analysis and investigation: [Tunc F. Ersoy, Daniel Brainman, Matthias Simon]; Writing - original draft preparation: [Tunc F. Ersoy]; Writing - review and editing: [Tunc F. Ersoy, Björn Berger, Florian Weissinger, Alexander Grote, Matthias Simon]; Resources: [Björn Berger, Roland Coras]; Supervision: [Matthias Simon].
Corresponding author
Ethics declarations
Ethical approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of Ärztekammer Westfalen-Lippe und der Westfälischen Wilhelms-Universität Münster, Germany (Az 2021-073-f-S).
Consent to participate
The responsible institutional research committee and local law does not require informed consent for this study.
Consent to publish
No personal data is published in this manuscript; there this is not applicable for this study.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ersoy, T.F., Brainman, D., Coras, R. et al. Defining the role of surgery for patients with multiple brain metastases. J Neurooncol (2024). https://doi.org/10.1007/s11060-024-04739-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11060-024-04739-7