Abstract
Purpose
Brain tumours are associated with neurocognitive impairments that are important for safe driving. Driving is vital to maintaining patient autonomy, despite this there is limited research on driving capacity amongst patients with brain tumours. The purpose of this review is to examine MVC risk in patients with brain tumours to inform development of clearer driving guidelines.
Methods
A systematic review was performed using Medline and EMBASE. Observational studies were included. The outcome of interest was MVC or measured risk of MVC in patients with benign or malignant brain tumours. Descriptive analysis and synthesis without meta-analysis were used to summarise findings. A narrative review of driving guidelines from Australia, United Kingdom and Canada was completed.
Results
Three studies were included in this review. One cohort study, one cross-sectional study and one case–control study were included (19,135 participants) across United States and Finland. One study evaluated the incidence of MVC in brain tumour patients, revealing no difference in MVC rates. Two studies measured MVC risk using driving simulation and cognitive testing. Patients found at higher risk of MVC had greater degrees of memory and visual attention impairments. However, predictive patient and tumour characteristics of MVC risk were heterogeneous across studies. Overall, driving guidelines had clear recommendations on selected conditions like seizures but were vague surrounding neurocognitive deficits.
Conclusion
Limited data exists regarding driving behaviour and MVC incidence in brain tumour patients. Existing guidelines inadequately address neurocognitive complexities in this group. Future studies evaluating real-world data is required to inform development of more applicable driving guidelines.
Systematic review registration number
PROSPERO 2023 CRD42023434608.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Driving is a complex task that requires intact neurocognitive skills including higher executive function, complex attention, visuospatial and motor function. Primary and metastatic brain tumours are associated with high morbidity and mortality, often requiring multimodality treatments including surgical resection, systemic and radiation therapy. As such, patients may be symptomatic from both disease and treatment. Seizures are a common complication of both primary and metastatic brain tumours and a major determinant of driving restrictions [1]. Approximately 80–90% of patients with diffuse gliomas will experience at least one seizure during their disease course [2, 3].
Motor vehicle crashes are a global public health issue. In Australia, there were 61,483 transport injuries (including car-occupied, motorcyclists, pedal cyclists and pedestrians) with 1,394 of these resulting in death between 2021 to 2022 [4]. In the US, there were roughly 36,355 deaths (1.21 death per registered motor vehicle related to motor vehicle crashes in 2019) [5]. Treating providers of patients with brain tumours have both a legal and ethical obligation in assessing patients’ fitness to drive. Despite this, they often have no formal training and lack resources and tools beyond guidelines that are formed on limited evidence base with utility limited to specific situational scenarios, such as seizures [6,7,8].
Driving is a crucial component of maintaining independence and quality of life. A longitudinal study revealed that patients with primary brain tumours attributed their loss of personal autonomy and day-to-day life problems to their inability to drive [9]. However, there remains little research exploring the driving capacity and implications of patients with brain tumours.
To our knowledge, there has not been a systematic review that has evaluated the relationship of driving with brain tumours and incidence of motor vehicle crashes (MVC). The aim of this study is to examine whether brain tumour patients are at higher risk of MVC. Addressing this question will inform the development of more comprehensive and clinically applicable driving guidelines for clinicians and patients.
Method
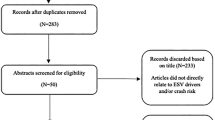
This study meets the requirements of the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement [10] (Systematic review registration number: PROSPERO 2023 CRD42023434608) (Fig. 1).
Eligibility criteria
Observational studies that were eligible for this systematic review included cohort studies, case-control studies and retrospective studies. The inclusion criteria were observational studies that were: reported in a peer-reviewed journal; the outcome of interest were MVC or measured risk of MVC by assessment (such as driving simulation, cognitive battery); and the exposure was driving with brain tumours including benign and malignant (primary and metastatic). The exclusion criteria were: case reports and case series; non-English studies; and studies of adults with history of paediatric intracranial tumours.
Search Strategy
Medline and Embase databases were searched using keywords related to brain tumour and MVC. Two reviewers (ST and AL) developed the search strategy. The search was developed and conducted in March 2023. The full search strategy and details of keywords used in the Medline database are reported in the Supplementary material. Time restriction was applied from 1974 to 7 March 2023.
Study selection
All eligible studies were compiled following the initial screening and duplicates removed. Two independent reviewers (ST and AL) reviewed all potential studies in three stages. Title and abstract screening of all records was performed initially. During this stage, if the eligibility of the study could not be determined, the article was retrieved for a full text review. Following title and abstract screening, full text articles of remaining records were screened. Additionally, the reference lists of included studies were also screened for further relevant articles that may have been missed on initial screening. Disagreements were resolved through discussions between reviewers.
Only national driving guidelines from English-speaking countries were included in this review. Guidelines from Australia, United Kingdom and Canada were accessed from each region’s driving board – Austroads and the National Transport Commission, Driver and Vehicle Licensing Agency and Canadian Medical Association respectively.
Data extraction
Data extracted from studies included: Study details (journal, publication year, study design); Patient details (age, sex, demographics, tumour location, tumour histopathology, symptoms of brain tumour, treatment type if applicable); Assessments of risk of MVC (type of cognitive assessments, driving simulation scenarios); and results (incidence of MVC, measured risk of MVC). Extracted data was corroborated by two reviewers (ST and AL). Study characteristics and interventions were tabulated for synthesis and comparison.
Evaluation of quality of studies
Quality of studies was independently assessed by two reviewers (ST and AL) using the Newcastle-Ottawa Scale (NOS) [11]. NOS is based on a star-scoring system that assesses studies on three domains – selection, comparability and outcome. Each study was scored from zero to nine stars. Studies rating 0 to 2 stars were considered to be poor, 3 to 5 fair and 6 to 9 high.
Data analysis
Due to the heterogeneity of study designs and outcome measures; a meta-analysis was not performed. Descriptive analysis and synthesis without meta-analysis were used to summarise findings. A narrative review of international driving guidelines was made.
Results
Study selection
The literature search identified 534 articles, of which 8 were duplicates and removed. The remaining 526 record abstracts and titles were screened with 465 records found to be irrelevant to the study question and thus excluded. Full-text reviews were undertaken of the remaining 61 records with 3 studies meeting all inclusion criteria and were included in the analysis. Among the 58 records excluded, 15 did not include adult patients with brain tumours, 13 did not have full texts available, two were not related to driving, one was not in English and 27 were case reports, literature reviews or letters to the editor.
Characteristics of studies included
The three eligible studies were published between 2018 and 2022 and included 19,135 participants from two countries, Finland and United States. The median age of participants was 54.3 years. All studies were conducted on both sexes.
The three studies included by Huuskonen et al., Mansur et al., and Estevis et al., all had different study designs; involving a cohort study, case-control study and cross-sectional study respectively (Table 1). Huuskonen et al., measured the incidence of MVC using a national road traffic safety database. Mansur et al., used a driving simulation test as a measure of MVC risk. Estevis et al., used the Cognitive Behavioural Driver’s Inventory (CBDI) as a surrogate marker of driving ability.
All studies were rated between five to six stars on the NOS and considered to be fair quality (Table 2). Estevis’ study performed the poorest in selection bias assessment and comparability. Regarding Huuskonen and Mansur’s studies, weaknesses concerned the selection of participants (outcome of interest not present at start of study, selection of controls) and outcome (follow-up not long enough for outcomes to occur and non-response rate).
Narrative synthesis
Huuskonen et al. (2022) [12]
The Finnish cohort study reviewed the incidence of MVC in a cancer cohort compared to a non-cancer cohort living in Southwest Finland from a nationwide motor registry. Among the 12,651 cancer and 6334 control patients included in the study, patients were divided into nine tumour streams. There were 452 patients with primary brain tumours identified, however only 154 patients were included as 66% were excluded due to license suspension. Though, the study has not specified the reason for license suspension, 40% of this group had experienced seizures. The primary endpoint was the first MVC leading to any injury and the secondary endpoint was MVC without injuries. Only MVCs of which patients were at-fault were included in the study. At a median follow-up of 53 months for control and 34 months for the cancer cohort, there were no events in the primary brain tumour cohort.. Overall, no significant difference in MVC risk was observed in the whole cancer group compared to controls (HR 0.89, [0.65–1.21]).
Mansur et al. (2018) [13]
This case-control study assessed MVC risk using a driving simulation and cognitive assessment. A self-reporting questionnaire on driving behaviours was also administered to 86 patients with brain tumours. The most common brain tumour types were pituitary adenomas (31%) and meningiomas (31%). Only seven patients had high grade malignant brain tumours. Most participants (70.9%) had surgical intervention, with the median time from surgery to survey completion being 36.3 months. Although approximately 40% of participants self-reported at least one collision since diagnosis, only one patient reported concerns with their driving behaviours, and 20% of participants limited their amount of driving from brain tumour diagnosis.
In the second part of the study, 26 participants (n = 13 brain tumour, n = 13 health controls) undertook the driving simulation and cognitive testing. The driving simulation included two experimental scenarios. The City Driving scenario involved driving in varying traffic conditions to assess ability to process and cope under higher cognitive demand. The ‘Bus following task’ required participants to follow a lead vehicle with changing speeds to assess attention and on-road adaptive ability. Brain tumour participants had significantly greater variability in speed than the control group (U = 57, p < 0.05) and twice the number of speed exceedances (U = 41, p < 0.95). There were no significant differences in the number of collisions in both scenarios. Tumour size and location did not correlate with driving performance. There was comparable executive function and visual attention between both groups, noting five patients (38%) had frontal lobe gliomas in the brain tumour group.
Estevis et al. (2019) [14]
This cross-sectional study retrospectively reviewed 64 patients with primary brain tumours who were referred to the Neuropsychology Service at the University of Texas for a clinical driving assessment between 2011 and 2016. The Cognitive Behavioural Driver’s Inventory (CBDI) comprised 28 performance measures assessed by computerised and written tasks and formed a surrogate measure of driving performance (Pass, Borderline or Fail). Baseline characteristics showed 47 patients (74%) had WHO Grade 3 or 4 tumours with majority (98%) managed with surgical resection followed by adjuvant chemoradiation (73%).
Forty-four (69%) participants passed, and twenty (31%) were borderline or did not pass; the latter was combined into one group.In the non-pass group, patients were older (62.6 years vs 49 years, P < 0.01), more likely to have tumours of the temporal lobe (60% vs 25%, P < 0.02) and higher number of WHO grade 4 tumours (95% vs 63%, P = 0.03). Logistic regression analysis showed age and tumour location to be the best predictor of CBDI outcome. The resulting R2 value of 0.25, suggests that age and tumour location explained 25% of the total variance of CBDI outcome (P = 0.005). Neuropsychological tests were also undertaken to assess cognitive domains important to safe driving. The greatest difference in performance between the pass/non-pass group was within the memory and visual attention domain.
Comparison of driving guidelines
Driving guidelines of English-speaking countries (Australia, United Kingdom and Canada) were reviewed. No formalised national guidelines regarding driving restrictions in patients with brain tumours exist in the United States.
The included guidelines varied in clarity and depth across different countries (Table 3). Most guidelines had clear recommendations for selected conditions such as seizures and post-intracranial surgery (immediate driving suspension). However, guidelines regarding neurocognitive deficits from intracranial tumours were less clear. The UK guidelines are most comprehensive regarding intracranial tumours, distinguishing benign and malignant tumours, as well as incorporating treatments modalities [7].
Despite distinguishing benign and malignant tumours, Canadian guidelines advised against generalised recommendations for malignant tumours, instead advocating for assessments by the patients’ treating neurologist or neurosurgeon [6]. Australian guidelines were predominantly situationally-focused with recommendations including immediate driving cessation post-intracranial surgery, with duration determined by the neurosurgeon and 6 to 12 months’ cessation for seizures depending upon risk factors [8]. All guidelines address visual deficits requiring acuity and field clearance but did not consistently address other neurological deficits.
Discussion
To our knowledge, this is the first systematic review evaluating driving in patients with brain tumours and incidence of MVC. To date, most published research has focused on clinician attitudes and practices towards driving guidelines [15,16,17,18,19]. Our review demonstrates a paucity of real-world data concerning MVC incidence amongst these patients. Of the three studies identified, only one study directly evaluated MVC incidence, rather than a surrogate marker. Whilst no significantly increased risk of MVC was reported by these studies Mansur et al. revealed that almost 40% of patients claimed to have had a MVC following diagnosis, suggesting discordance between real-world data and the driving risk assessment tools used [13]. Data interpretation is limited by small sample size, lack of a control population, heterogenous tumour types and selection bias surrounding coexistence with seizures. Nevertheless, all studies underline the neurological complexities involved in safe driving and suggest the need for more comprehensive and specific cognitive assessments and guidelines for patients driving with brain tumours.
The responsibility of determining a patient’s medical fitness to drive often falls on the treating clinician. However, surveys globally have consistently highlighted that most clinicians are unaware of driving guidelines [15,16,17,18,19,20,21]. In an Australian survey of 194 practicing neurosurgeons, radiation oncologists and neurologists, 73% of respondents were not aware of guidelines and only 38% of doctors discussed driving restrictions with patients [20]. North American studies have reported similar findings [15, 17, 18].
Additionally, providers familiar with driving guidelines, report significant heterogeneity in their application [20]. A Canadian study evaluating driving recommendations among healthcare professionals, found that only 56% discussed driving recommendations with patients and only 9.3% could reliably determine fitness to drive [18]. In a survey of Australian and New Zealand palliative care clinicians, only 27% reported patients with brain tumours to driving authorities [19]. Similarly, a Canadian study demonstrated low mandatory reporting rates with poor documentation of medical discussions regarding safe driving [15]. There are likely several factors that contribute to this diverse practice and reluctance to report, including lack of awareness of guidelines, limited tools and training to determine fitness-to-drive, and intent to preserve patients’ autonomy [22]. Altogether, these findings re-affirm the need to develop more comprehensive and specific driving recommendations at a global level.
Furthermore, driving recommendations from governing authorities do not address the clinical issues patients with brain tumours typically face. Specific guidelines addressing seizures and time since surgery exist, but there is little emphasis on impaired neurocognitive and motor functions from disease progression and treatment toxicities. A pilot study of neurocognitive function following radiation therapy to one to three brain metastases revealed baseline deficits in learning/memory, motor dexterity and higher executive function [23]. Additionally, one month after stereotactic radiosurgery, 54% of patients declined in two or more domains; especially motor dexterity and learning/memory. Thus, whilst current guidelines offer clear recommendations for specific situations such as seizures, they inadequately address neurocognitive deficits related to disease progression or treatment toxicities.
Aside from UK guidelines, Australian and Canadian guidelines do not consider actual tumour characteristics. Though the studies by Mansur et al. and Estevis et al. did not find correlation between tumour histology or location with driving performance; studies have shown some difference in symptomatology and risk of progression that may impact on MVC risk. Lower grade gliomas have higher frequency of seizures; conversely malignant tumours have lower incidences due to its rapid and destructive growth [2, 3]. Tumour location can impact on syndrome of neurological deficits, such as posterior fossa tumours which are associated with cerebellar signs, cranial nerve palsies and raised intracranial pressures [24]. As such, these factors need to be reflected in driving guidelines.
Driving is complex, involving several neurocognitive domains and therefore requires a multidisciplinary approach to support clinicians in balancing competing patient advocacy and community safety responsibilities. As demonstrated in Mansur’s study, no single cognitive test can predict driving performance [13]. A meta-analysis of driving assessments of patients with Alzheimer’s dementia and mild cognitive impairment show that measures of cognitive and sensory domains may be predictive of driving performance with the TMT test (assess attention, processing speed and adaptability) and the Maze Task (measures executive planning and visuospatial awareness) being better predictors of on-road performance than driving simulations [25]. Mansur’s study included cognitive assessments with both tests, however they were not predictive of MVC. Therefore, cognitive testing alone in assessments of ‘fitness-to-drive’ is inadequate. This further highlights the need for a more comprehensive guide and assessment for clinicians on evaluating patient’s fitness-to-drive.
To address the gap in driving assessment tools, the Swiss Neuro-Oncology Society in collaboration with the Swiss Society for Legal Medicine have developed a guideline involving routine brain MRI, thorough history, neuropsychological and visual assessments to determine ‘fitness-to-drive’ in patients with glioblastoma following their initial treatment [26]. This group is now conducting a pilot project prospectively recruiting patients with glioblastoma who wish to return to driving, to determine feasibility of this proposed tool in daily clinical practice and to match outcomes with events from the Road Traffic Registry (GLIODRIVE). Beyond this and perhaps more importantly, is the development of driver rehabilitation programs to re-introduce patients to driving.
Several limitations should be considered of this review. Few studies of fair quality were included. These studies were heterogeneous in design, methodology and outcomes, making comparisons challenging and limiting conclusions drawn. Additionally sample sizes across the three studies were small. Another major limitation of all three studies is that they do not consider the dynamic course of brain tumours, and the risk of disease progression and thus, development of new deficits or complications. This is important to consider when developing guidelines and assessment frameworks. Whilst the gold standard test for assessing driving ability is an on-road driving assessment, only one study looked at real-world driving outcomes.
Future research should include registry studies that examine real-world data of impact of brain tumours on MVC risk and explore what factors may increase MVC risk such as disease characteristics (tumour histopathology, location), treatment modalities and patient characteristics. Studies examining neurocognitive domains important to safe driving and how they are affected in brain tumours is also important to guide development of driving assessment tools. Addressing these gaps in literature is required to form evidence-based driving recommendations.
Conclusion
Overall, there is limited data regarding MVC risk and driving behaviours in patients driving with brain tumours.
Though there was no significant difference in MVC risk observed in the studies included, our review has highlighted neurocognitive deficits in domains important to safe driving in this group. Current driving guidelines inadequately address these deficits and do not reflect the dynamic nature of disease with progression and treatment toxicities. Further work is required to explore driving behaviours and risk in patients with brain tumours to guide formation of driving assessment tools and frameworks to allow for development of more comprehensive guidelines that balance patient autonomy and community safety.
Data availability
No datasets were generated or analysed during the current study.
References
Avila EK, Graber J (2010) Seizures and epilepsy in cancer patients. Curr Neurol Neurosci Rep 10(1):60–67
van Breemen MS, Rijsman RM, Taphoorn MJ, Walchenbach R, Zwinkels H, Vecht CJ (2009) Efficacy of anti-epileptic drugs in patients with gliomas and seizures. J Neurol 256(9):1519–1526
Pallud J, Audureau E, Blonski M, Sanai N, Bauchet L, Fontaine D et al (2014) Epileptic seizures in diffuse low-grade gliomas in adults. Brain 137(Pt 2):449–462
Australian Institute of Health and Welfare (2023) Transport accidents [Internet]. Canberra: Australian Institute of Health and Welfare, [cited 2023 Oct. 25]. Available from: https://www.aihw.gov.au/reports/injury/transport-accidents. Accessed 25 Oct 2023
Yellman MA, Sauber-Schatz EK (2022) Motor Vehicle Crash Deaths - United States and 28 Other High-Income Countries, 2015 and 2019. MMWR Morb Mortal Wkly Rep 71(26):837–843
Canadian Medical Association (2019) Determining Medical Fitness to Operate Motor Vehicles. CMA Driver’s Guide, 9th edn. CMA, Ottawa
Driver and Vehicle Licensing Agency (2022) Assessing fitness to drive—a guide for medical professionals. DVLA, London
Austroads and the National Transport Commission (2022) Assessing fitness to drive for commercial and private vehicle drivers: medical standards for licensing and clinical management guidelines. NTC, Sydney
Molassiotis A, Wilson B, Brunton L, Chaudhary H, Gattamaneni R, McBain C (2010) Symptom experience in patients with primary brain tumours: a longitudinal exploratory study. Eur J Oncol Nurs 14(5):410–416
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP et al (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 339:b2700
Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, Tugwell P, luchini C, Stubbs B, Solmi (2014) The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed 10 Oct 2023
Huuskonen ML, Koistinen T, Sihvola N, Parkkari I, Palovaara S, Kyto V et al (2023) Controlled register-based study of road traffic accidents in 12,651 Finnish cancer patients during 2013–2019. Cancer Med 12(6):7406–7413
Mansur A, Hird MA, Desimone A, Pshonyak I, Schweizer TA, Das S (2018) Driving habits and behaviors of patients with brain tumors: a self-report, cognitive and driving simulation study. Sci Rep 8(1):4635
Estevis E, Noll KR, Bradshaw ME, Wefel JS (2019) Driver safety in patients with primary brain tumors. Neurooncol Pract 6(6):490–498
Louie A, Chan E, Hanna M, Bauman G, Fisher B, Palma D et al (2013) Assessing fitness to drive in brain tumour patients: a grey matter of law, ethics, and medicine. Curr Oncol 20(2):90–96
Louie A, D’Souza D, Palma D, Bauman G, Lock M, Fisher B et al (2012) Fitness to drive in patients with brain tumours: the influence of mandatory reporting legislation on radiation oncologists in Canada. Curr Oncol 19(3):117–122
Chan E, Louie AV, Hanna M, Bauman GS, Fisher BJ, Palma DA et al (2013) Multidisciplinary assessment of fitness to drive in brain tumour patients in southwestern Ontario: a grey matter. Curr Oncol 20(1):e4–e12
Mansur A, Desimone A, Vaughan S, Schweizer TA, Das S (2018) To drive or not to drive, that is still the question: current challenges in driving recommendations for patients with brain tumours. J Neurooncol 137(2):379–385
Weir NFA, Good P (2017) Assessing the practice of Palliative Care Doctors – What driving advice do they give patients with advanced disease? RACP Intern Med J 47(10):1161–1165
Chin Y, Jayamohan J, Clouston P, Gebski V, Cakir B (2004) Driving and patients with brain tumours: a postal survey of neurosurgeons, neurologists and radiation oncologists. J Clin Neurosci 11(5):471–474
Thomas S, Mehta MP, Kuo JS, Ian Robins H, Khuntia D (2011) Current practices of driving restriction implementation for patients with brain tumors. J Neurooncol 103(3):641–647
Redelmeier DA, Vinkatesh V, Stanbrook MB (2008) Mandatory reporting by physicians of patients potentially unfit to drive. Open Med 2(1):e8–e17
Chang EL, Wefel JS, Maor MH, Hassenbusch SJ 3rd, Mahajan A, Lang FF et al (2007) A pilot study of neurocognitive function in patients with one to three new brain metastases initially treated with stereotactic radiosurgery alone. Neurosurgery 60(2):277–83 (discussion 83-4)
Rees JH (2011) Diagnosis and treatment in neuro-oncology: an oncological perspective. Br J Radiol 84 Spec No 2(Spec Iss 2):S82-9
Hird MA, Egeto P, Fischer CE, Naglie G, Schweizer TA (2016) A systematic review and meta-analysis of on-road simulator and cognitive driving assessment in Alzheimer’s disease and mild cognitive impairment. J Alzheimers Dis 53(2):713–729
Hofer S, Keller K, Imbach L, Roelcke U, Hutter G, Hundsberger T et al (2021) Fitness-to-drive for glioblastoma patients: Guidance from the Swiss Neuro-Oncology Society (SwissNOS) and the Swiss Society for Legal Medicine (SGRM). Swiss Med Wkly 151:w20501
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. The authors declare that no funds, grants, or other supports were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
Study conception and design was by M.A The search strategy was designed by S.T and A.L. Data collection and analysis were performed by S.T and A.L. The first draft of the manuscript was written by S.T and all authors have provided feedback and commentary on previous versions of the manuscript. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tran, S., Lapidus, A., Neal, A. et al. A systematic review of the impact of brain tumours on risk of motor vehicle crashes. J Neurooncol 166, 395–405 (2024). https://doi.org/10.1007/s11060-024-04586-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-024-04586-6