Abstract
Purpose
Glioblastomas, the most common primary malignant brain tumors in adults, still hold poor prognosis. Corticosteroids, such as dexamethasone, are usually prescribed to reduce peritumoral edema and limit neurological symptoms, although potential detrimental effects of these drugs have been described. The present meta-analysis aimed to explore the association of dexamethasone with overall survival (OS) and progression free survival (PFS) in patients with newly diagnosed glioblastoma.
Methods
PubMed, Cochrane Library, Embase, and ClinicalTrials.gov were searched for pertinent studies following the Preferred Reporting Items of Systematic Review and Meta-Analysis checklist. Pooled multivariable-adjusted hazard ratios (HR) for OS and PFS and their associated 95% confidence intervals (CIs) were calculated using the random-effects model and the heterogeneity among studies was assessed using I2. The quality of evidence was assessed using the GRADE criteria.
Results
Seven studies were included, pooling data of 1,257 patients, with age varying from 11 to 81 years. Glioblastoma patients on pre- or peri-operative dexamethasone were associated with a significantly poorer overall survival (HR: 1.33, 95% CI: 1.15, 1.55; 7 studies; I2: 59.9%) and progression free survival (HR: 1.77, 95% CI: 1.05, 2.97; 3 studies; I2: 71.1%) compared to patients not on dexamethasone. The quality of evidence was moderate for overall survival and low for progression free survival.
Conclusion
Dexamethasone appeared to be associated with poor survival outcomes of glioblastoma patients.



Similar content being viewed by others
Data availability
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials; additional information can be requested from the corresponding author, R.M.
References
Delgado-Lopez PD, Corrales-Garcia EM (2016) Survival in glioblastoma: a review on the impact of treatment modalities. Clin Transl Oncol 18:1062–1071. https://doi.org/10.1007/s12094-016-1497-x
Balana C, Vaz MA, Manuel Sepulveda J, Mesia C, Del Barco S, Pineda E, Munoz-Langa J, Estival A, de Las Penas R, Fuster J, Girones R, Navarro LM, Gil-Gil M, Alonso M, Herrero A, Peralta S, Olier C, Perez-Segura P, Covela M, Martinez-Garcia M, Berrocal A, Gallego O, Luque R, Perez-Martin FJ, Esteve A, Munne N, Domenech M, Villa S, Sanz C, Carrato C (2020) A phase II randomized, multicenter, open-label trial of continuing adjuvant temozolomide beyond 6 cycles in patients with glioblastoma (GEINO 14 – 01). Neuro Oncol 22:1851–1861. https://doi.org/10.1093/neuonc/noaa107
Stupp R, Brada M, van den Bent MJ, Tonn JC, Pentheroudakis G, Group EGW (2014) High-grade glioma: ESMO Clinical Practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 25(Suppl 3):iii93–101. https://doi.org/10.1093/annonc/mdu050
Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO, European Organisation for R, Treatment of Cancer Brain T, Radiotherapy G, National Cancer Institute of Canada Clinical Trials G (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352: 987–996 https://doi.org/10.1056/NEJMoa043330
Liu S, Shi W, Zhao Q, Zheng Z, Liu Z, Meng L, Dong L, Jiang X (2021) Progress and prospect in Tumor treating fields treatment of glioblastoma. Biomed Pharmacother 141:111810. https://doi.org/10.1016/j.biopha.2021.111810
Weller M, van den Bent M, Preusser M, Le Rhun E, Tonn JC, Minniti G, Bendszus M, Balana C, Chinot O, Dirven L, French P, Hegi ME, Jakola AS, Platten M, Roth P, Ruda R, Short S, Smits M, Taphoorn MJB, von Deimling A, Westphal M, Soffietti R, Reifenberger G, Wick W (2021) EANO guidelines on the diagnosis and treatment of diffuse gliomas of adulthood. Nat Rev Clin Oncol 18:170–186. https://doi.org/10.1038/s41571-020-00447-z
Guo X, Yang X, Wu J, Yang H, Li Y, Li J, Liu Q, Wu C, Xing H, Liu P, Wang Y, Hu C, Ma W (2022) Tumor-treating fields in Glioblastomas: past, Present, and Future. Cancers (Basel) 14. https://doi.org/10.3390/cancers14153669
Stupp R, Taillibert S, Kanner AA, Kesari S, Steinberg DM, Toms SA, Taylor LP, Lieberman F, Silvani A, Fink KL, Barnett GH, Zhu JJ, Henson JW, Engelhard HH, Chen TC, Tran DD, Sroubek J, Tran ND, Hottinger AF, Landolfi J, Desai R, Caroli M, Kew Y, Honnorat J, Idbaih A, Kirson ED, Weinberg U, Palti Y, Hegi ME, Ram Z (2015) Maintenance Therapy with Tumor-Treating Fields Plus Temozolomide vs Temozolomide alone for Glioblastoma: a Randomized Clinical Trial. JAMA 314:2535–2543. https://doi.org/10.1001/jama.2015.16669
Kostaras X, Cusano F, Kline GA, Roa W, Easaw J (2014) Use of dexamethasone in patients with high-grade glioma: a clinical practice guideline. Curr Oncol 21:e493–503. https://doi.org/10.3747/co.21.1769
Ly KI, Wen PY (2017) Clinical relevance of Steroid Use in Neuro-Oncology. Curr Neurol Neurosci Rep 17:5. https://doi.org/10.1007/s11910-017-0713-6
Schiff D, Lee EQ, Nayak L, Norden AD, Reardon DA, Wen PY (2015) Medical management of brain tumors and the sequelae of treatment. Neuro Oncol 17:488–504. https://doi.org/10.1093/neuonc/nou304
Pitter KL, Tamagno I, Alikhanyan K, Hosni-Ahmed A, Pattwell SS, Donnola S, Dai C, Ozawa T, Chang M, Chan TA, Beal K, Bishop AJ, Barker CA, Jones TS, Hentschel B, Gorlia T, Schlegel U, Stupp R, Weller M, Holland EC, Hambardzumyan D (2016) Corticosteroids compromise survival in glioblastoma. Brain 139:1458–1471. https://doi.org/10.1093/brain/aww046
Wong ET, Lok E, Gautam S, Swanson KD (2015) Dexamethasone exerts profound immunologic interference on treatment efficacy for recurrent glioblastoma. Br J Cancer 113:232–241. https://doi.org/10.1038/bjc.2015.238
Zhou L, Shen Y, Huang T, Sun Y, Alolga RN, Zhang G, Ge Y (2021) The Prognostic Effect of Dexamethasone on patients with glioblastoma: a systematic review and Meta-analysis. Front Pharmacol 12:727707. https://doi.org/10.3389/fphar.2021.727707
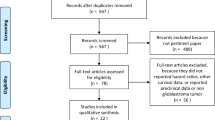
Moher D, Liberati A, Tetzlaff J, Altman DG, Group P (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6:e1000097. https://doi.org/10.1371/journal.pmed.1000097
Cenciarini M, Valentino M, Belia S, Sforna L, Rosa P, Ronchetti S, D’Adamo MC, Pessia M (2019) Dexamethasone in Glioblastoma Multiforme Therapy: mechanisms and controversies. Front Mol Neurosci 12:65. https://doi.org/10.3389/fnmol.2019.00065
Innovation VH Covidence systematic review software
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7:177–188. https://doi.org/10.1016/0197-2456(86)90046-2
Cochran WG (1950) The comparison of percentages in Matched samples. Biometrika 37. https://doi.org/10.2307/2332378
Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327:557–560. https://doi.org/10.1136/bmj.327.7414.557
Wells GS, O’Connell B, Peterson D, Welch J, Losos V, Tugwell M (2013) P The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck-Ytter Y, Schunemann HJ, Group GW (2008) What is quality of evidence and why is it important to clinicians? BMJ 336:995–998. https://doi.org/10.1136/bmj.39490.551019.BE
Begg CB, Mazumdar M (1994) Operating characteristics of a rank correlation test for publication bias. Biometrics 50:1088–1101
Bhavsar S, Hagan K, Arunkumar R, Potylchansky Y, Grasu R, Dang A, Carlson R, Cowels C, Arnold B, Rahlfs TF, Lipski I, Walsh C, Nguyen AT, Feng L, Cata JP (2016) Preoperative statin use is not associated with improvement in survival after glioblastoma Surgery. J Clin Neurosci 31:176–180. https://doi.org/10.1016/j.jocn.2016.03.010
Derr RL, Ye X, Islas MU, Desideri S, Saudek CD, Grossman SA (2009) Association between hyperglycemia and survival in patients with newly diagnosed glioblastoma. J Clin Oncol 27:1082–1086. https://doi.org/10.1200/JCO.2008.19.1098
Dubinski D, Won SY, Gessler F, Quick-Weller J, Behmanesh B, Bernatz S, Forster MT, Franz K, Plate KH, Seifert V, Harter PN, Senft C (2018) Dexamethasone-induced leukocytosis is associated with poor survival in newly diagnosed glioblastoma. J Neurooncol 137:503–510. https://doi.org/10.1007/s11060-018-2761-4
Hui CY, Rudra S, Ma S, Campian JL, Huang J (2019) Impact of overall corticosteroid exposure during chemoradiotherapy on lymphopenia and survival of glioblastoma patients. J Neurooncol 143:129–136. https://doi.org/10.1007/s11060-019-03146-7
Lee C, Ahn S, Park JS, Song JH, Hong YK, Jeun SS (2020) Effect of cumulative dexamethasone dose during concomitant chemoradiation on Lymphopenia in patients with newly diagnosed Glioblastoma. Brain Tumor Res Treat 8:71–76. https://doi.org/10.14791/btrt.2020.8.e12
Lewitzki V, Klement RJ, Kosmala R, Lisowski D, Flentje M, Polat B (2019) Accelerated hyperfractionated radiochemotherapy with temozolomide is equivalent to normofractionated radiochemotherapy in a retrospective analysis of patients with glioblastoma. Radiat Oncol 14:227. https://doi.org/10.1186/s13014-019-1427-5
Shields LB, Shelton BJ, Shearer AJ, Chen L, Sun DA, Parsons S, Bourne TD, LaRocca R, Spalding AC (2015) Dexamethasone administration during definitive radiation and temozolomide renders a poor prognosis in a retrospective analysis of newly diagnosed glioblastoma patients. Radiat Oncol 10:222. https://doi.org/10.1186/s13014-015-0527-0
Petrelli F, De Stefani A, Ghidini A, Bruschieri L, Riboldi V, Dottorini L, Iaculli A, Zaniboni A, Trevisan F (2021) Steroids use and survival in patients with Glioblastoma Multiforme: a pooled analysis. J Neurol 268:440–447. https://doi.org/10.1007/s00415-020-09731-5
Ostrom QT, Gittleman H, Farah P, Ondracek A, Chen Y, Wolinsky Y, Stroup NE, Kruchko C, Barnholtz-Sloan JS (2013) CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2006–2010. Neuro Oncol 15(Suppl 2):ii1–56. https://doi.org/10.1093/neuonc/not151
Jelsma R, Bucy PC (1967) The treatment of Glioblastoma Multiforme of the brain. J Neurosurg 27:388–400. https://doi.org/10.3171/jns.1967.27.5.0388
Michinaga S, Koyama Y (2015) Pathogenesis of brain edema and investigation into anti-edema Drugs. Int J Mol Sci 16:9949–9975. https://doi.org/10.3390/ijms16059949
Qin X, Liu R, Akter F, Qin L, Xie Q, Li Y, Qiao H, Zhao W, Jian Z, Liu R, Wu S (2021) Peri-tumoral brain edema associated with glioblastoma correlates with Tumor recurrence. J Cancer 12:2073–2082. https://doi.org/10.7150/jca.53198
Gu YT, Qin LJ, Qin X, Xu F (2009) The molecular mechanism of dexamethasone-mediated effect on the blood-brain Tumor barrier permeability in a rat Brain Tumor model. Neurosci Lett 452:114–118. https://doi.org/10.1016/j.neulet.2008.12.047
Gu YT, Xue YX, Wang P, Zhang H, Qin LJ, Liu LB (2009) Dexamethasone enhances calcium-activated potassium channel expression in blood-brain Tumor barrier in a rat Brain Tumor model. Brain Res 1259:1–6. https://doi.org/10.1016/j.brainres.2008.12.080
Hue CD, Cho FS, Cao S, Dale Bass CR, Meaney DF, Morrison B 3rd (2015) Dexamethasone potentiates in vitro blood-brain barrier recovery after primary blast injury by glucocorticoid receptor-mediated upregulation of ZO-1 tight junction protein. J Cereb Blood Flow Metab 35:1191–1198. https://doi.org/10.1038/jcbfm.2015.38
Gu YT, Zhang H, Xue YX (2007) Dexamethasone enhances adenosine 5’-triphosphate-sensitive potassium channel expression in the blood-brain Tumor barrier in a rat Brain Tumor model. Brain Res 1162:1–8. https://doi.org/10.1016/j.brainres.2007.05.053
Fan Z, Sehm T, Rauh M, Buchfelder M, Eyupoglu IY, Savaskan NE (2014) Dexamethasone alleviates tumor-associated brain damage and angiogenesis. PLoS ONE 9:e93264. https://doi.org/10.1371/journal.pone.0093264
Liu H, Huang X, Wang H, Shen A, Cheng C (2009) Dexamethasone inhibits proliferation and stimulates SSeCKS expression in C6 rat glioma cell line. Brain Res 1265:1–12. https://doi.org/10.1016/j.brainres.2009.01.050
Sur P, Sribnick EA, Patel SJ, Ray SK, Banik NL (2005) Dexamethasone decreases temozolomide-induced apoptosis in human gliobastoma T98G cells. Glia 50:160–167. https://doi.org/10.1002/glia.20168
Wang H, Li M, Rinehart JJ, Zhang R (2004) Pretreatment with dexamethasone increases antitumor activity of carboplatin and gemcitabine in mice bearing human cancer xenografts: in vivo activity, pharmacokinetics, and clinical implications for cancer chemotherapy. Clin Cancer Res 10:1633–1644. https://doi.org/10.1158/1078-0432.ccr-0829-3
Lin YM, Jan HJ, Lee CC, Tao HY, Shih YL, Wei HW, Lee HM (2008) Dexamethasone reduced invasiveness of human malignant glioblastoma cells through a MAPK phosphatase-1 (MKP-1) dependent mechanism. Eur J Pharmacol 593:1–9. https://doi.org/10.1016/j.ejphar.2008.06.111
Piette C, Deprez M, Roger T, Noel A, Foidart JM, Munaut C (2009) The dexamethasone-induced inhibition of proliferation, migration, and invasion in glioma cell lines is antagonized by macrophage migration inhibitory factor (MIF) and can be enhanced by specific MIF inhibitors. J Biol Chem 284:32483–32492. https://doi.org/10.1074/jbc.M109.014589
Guan Y, Chen J, Zhan Y, Lu H (2018) Effects of dexamethasone on C6 cell proliferation, migration and invasion through the upregulation of AQP1. Oncol Lett 15:7595–7602. https://doi.org/10.3892/ol.2018.8269
Dietrich J, Rao K, Pastorino S, Kesari S (2011) Corticosteroids in Brain cancer patients: benefits and pitfalls. Expert Rev Clin Pharmacol 4:233–242. https://doi.org/10.1586/ecp.11.1
Petrillo MG, Fettucciari K, Montuschi P, Ronchetti S, Cari L, Migliorati G, Mazzon E, Bereshchenko O, Bruscoli S, Nocentini G, Riccardi C (2014) Transcriptional regulation of kinases downstream of the T cell receptor: another immunomodulatory mechanism of glucocorticoids. BMC Pharmacol Toxicol 15:35. https://doi.org/10.1186/2050-6511-15-35
Jabara HH, Brodeur SR, Geha RS (2001) Glucocorticoids upregulate CD40 ligand expression and induce CD40L-dependent immunoglobulin isotype switching. J Clin Invest 107:371–378. https://doi.org/10.1172/JCI10168
Pearson JRD, Cuzzubbo S, McArthur S, Durrant LG, Adhikaree J, Tinsley CJ, Pockley AG, McArdle SEB (2020) Immune Escape in Glioblastoma Multiforme and the adaptation of immunotherapies for treatment. Front Immunol 11:582106. https://doi.org/10.3389/fimmu.2020.582106
Jatana S, Mohammad AH, Al-Saadi TD, Carias M, Guevara-Moriones N, Ruiz-Barrera MA, Mindru CS, Diaz RJ (2023) Characterization of perioperative glycemic status and dexamethasone use with associated postoperative Complications in glioblastoma patients. Acta Neurochir (Wien) 165:1031–1040. https://doi.org/10.1007/s00701-023-05541-6
Li S, Dong J, Wang X, Meng X, Jiang C, Cai J (2022) Dexamethasone and compliance affect TTFields efficacy to glioblastoma patients: a systematic review and meta-analysis. Chin Neurosurg J 8:24. https://doi.org/10.1186/s41016-022-00294-0
Linder B, Schiesl A, Voss M, Rodel F, Hehlgans S, Gullulu O, Seifert V, Kogel D, Senft C, Dubinski D (2021) Dexamethasone treatment limits efficacy of Radiation, but does not interfere with Glioma Cell Death Induced by Tumor Treating fields. Front Oncol 11:715031. https://doi.org/10.3389/fonc.2021.715031
Caramanna I, de Kort JM, Brandes AA, Taal W, Platten M, Idbaih A, Frenel JS, Wick W, Preetha CJ, Bendszus M, Vollmuth P, Reijneveld JC, Klein M (2022) Corticosteroids use and neurocognitive functioning in patients with recurrent glioblastoma: evidence from European Organization for Research and Treatment of Cancer (EORTC) trial 26101. Neurooncol Pract 9:310–316. https://doi.org/10.1093/nop/npac022
El-Adawy AME-S, Mohamed KAK, Kelany MR, Abdelrahman OM (2018) Retrospective study of the Corticosteroids Administration in Glioblastoma patients as a prognostic factor in the Disease. Egypt J Hosp Med 72:4551–4555. https://doi.org/10.21608/ejhm.2018.9544
Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D, Hawkins C, Ng HK, Pfister SM, Reifenberger G, Soffietti R, von Deimling A, Ellison DW (2021) The 2021 WHO classification of tumors of the Central Nervous System: a summary. Neuro Oncol 23:1231–1251. https://doi.org/10.1093/neuonc/noab106
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
H.A. and M.M. wrote the main manuscript text; N.P., D.Z., and H.D. were responsible of investigation and formal analysis; I.Y. and T.S. provided edits to the analysis and to the manuscript; T.S. conceptualized and designed the study; R.M. assisted in all stages of the study, from conceptualization and definition of methodology, to data analysis and manuscript editing. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests. The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Arora, H., Mammi, M., Patel, N.M. et al. Dexamethasone and overall survival and progression free survival in patients with newly diagnosed glioblastoma: a meta-analysis. J Neurooncol 166, 17–26 (2024). https://doi.org/10.1007/s11060-023-04549-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-023-04549-3