Abstract
Purpose
To perform a systematic review of literature specific to single-fraction stereotactic radiosurgery (SRS) for large vestibular schwannomas (VS), maximum diameter ≥ 2.5 cm and/or classified as Koos Grade IV, and to present consensus recommendations on behalf of the International Stereotactic Radiosurgery Society (ISRS).
Methods
The Medline and Embase databases were used to apply the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) approach. We considered eligible prospective and retrospective studies, written in the English language, reporting treatment outcomes for large VS; SRS for large post-operative tumors were analyzed in aggregate and separately.
Results
19 of the 229 studies initially identified met the final inclusion criteria. Overall crude rate of tumor control was 89% (93.7% with no prior surgery vs 87.7% with prior surgery). Rates of salvage microsurgical resection, need for shunt, and additional SRS in all series versus those with no prior surgery were 9.6% vs 3.3%, 4.7% vs 6.4% and 1% vs 0.9%, respectively. Rates of facial palsy and hearing preservation in all series versus those with no prior surgery were 1.3% vs 3.4% and 34.2% vs 40.4%, respectively.
Conclusions
Upfront SRS resulted in high rates of tumor control with acceptable rates of facial palsy and hearing preservation as compared to the results in those series including patients with prior surgery (level C evidence). Therefore, although large VS are considered classic indication for microsurgical resection, upfront SRS can be considered in selected patients and we recommend a prescribed marginal dose from 11 to 13 Gy (level C evidence).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Stereotactic radiosurgery (SRS) is a widely accepted treatment for small to medium sized vestibular schwannomas (Koos grades I, II and III) [1, 2]. However, for large vestibular schwannoma (VS), using a definitional threshold of ≥ 2.5 cm or Koos Grade IV designation, most recommend microsurgical resection [3]. In particular, surgery should be considered when there are signs and symptoms of mass effect related to brainstem compression, cranial nerve (CN) neuropathy (other than CN VIII and particularly CN V), and/or presence of hydrocephalus [4]. Importantly, a wait and scan strategy is usually not recommended due to the possibility of life-threatening complications associated with tumor progression [5].
For a patient with a large VS who is not an optimal candidate for microsurgical resection, some type of fractionated radiation therapy is typically recommended. However, several centers have treated these patients with single fraction SRS, as they would do for smaller VS [6, 7]. Concerns of single-fraction SRS in these patients range from development of serious adverse radiation events (ARE), transient-tumor-expansion (TTE, also referred to as pseudoprogression), delayed time-to-response for patients who have symptomatic hydrocephalus, and late treatment failure necessitating surgery which may put the patient at an increased risk of surgical complications [8]. To date, there has yet to be a critical review of the published literature specific to this population to define efficacy and toxicity of this approach. Therefore, the purpose of this systematic review is to summarize the current literature specific to single fraction SRS for large VS, and provide treatment recommendations on behalf of the International Stereotactic Radiosurgery Society (ISRS) Guidelines Committee.
Methods
Systematic review
A systematic review of the literature was performed using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) approach [9].
Search strategy
A search strategy evaluated the Medline and Embase databases to search for articles published from 1968 to June 2022. The following MESH terms or combination of those were used either in title/abstract: “radiosurgery” AND “vestibular schwannomas” AND “large” OR “Koos IV.”
Inclusion criteria
We included prospective and retrospective studies, written in the English language, reporting patients treated for a large VS with either upfront single fractions SRS or those treated with single fraction SRS following surgery to a residual or recurrent tumor. We abstracted data for large VS based on those tumors with a maximum diameter ≥ 2.5 cm and/or classified as Koos Grade IV (large tumors with brainstem and cranial nerve displacement) [10].
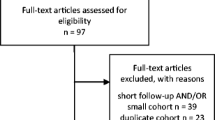
We initially identified 229 studies, of which after screening abstracts, 120 were excluded (Fig. 1). The remaining 109 studies were further screened with a detailed review of the published manuscript. We retained only those 19 articles that met our strict inclusion and exclusion criteria[6, 8, 11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26]. 6/19 reported outcomes in patients who had not been previously resected[11, 15, 19, 23, 27, 28], and the remaining 13/19 studies included patients with prior surgical resection[6, 8, 12,13,14, 16,17,18, 21, 22, 25, 26]. Of note, the multicenter study by Pikis et al.[28] was kept in our analysis given the large number of cases and updated patient outcome information, despite potential overlap with single institution studies from those centers participating in the multicenter cohort. Demographic data are summarized in Table 1, and Table 2 summarizes dosimetric statistics and target volumes.
Exclusion criteria
We excluded studies written in languages other than English, duplicate studies from the same author or institution, and studies reporting fractionated stereotactic radiotherapy outcomes[15]. Those reports identified in the initial search strategy that included combined outcomes with either SRS or fractionated stereotactic radiotherapy were excluded if outcomes specific to the SRS cohort could not be segregated.
Outcome measures
The primary outcome for this analysis was the radiographic local tumor control rate, however, the definition of tumor control varied considerably amongst publications. As an example, Chung et al.[12] considered “tumor regression” if the post-treatment volume was less than 100% of the pre-SRS volume, “stable disease” if the post-treatment volume was within 100–110% of the pre-SRS volume, and “disease progression” if the post-treatment volume was > 110% of the pre-SRS volume. In the largest study by Pikis et al.[28], local failure was defined as an increase in the total VS volume of more than 20% at last follow-up, while decrease was defined as a reduction in tumor volume of more than 20% from baseline at the last radiological follow-up. Given the heterogeneity in the definition of local control across studies and to facilitate summary statistics, we defined radiological local control as stability or a decrease in tumor volume at last follow-up (regardless of the degree of tumor reduction). We also considered treatment failure in those patients with tumor enlargement. Further microsurgical interventions, surgical management of hydrocephalus, delivery of further SRS, cystic puncture etc., were noted for the adverse event analyses and not counted as a treatment failure (Table 2). Adverse clinical outcomes specific to CN toxicities were summarized separately.
Follow-up periods are illustrated in Table 1 and are heterogeneous.
Establishment of evidence based guidelines
The present systematic review has been performed by a group of international experts from a wide range of disciplines. Evidence was gathered from the primary literature. Recommendations, which are further summed, were made on the basis of this evidence and were graded in terms of their strength.
Results
Tumor control
Tumor control (stability or decrease)
The overall tumor control in all series was 89.0% (range 86.1–91.9%, I2 = 56.28%, p heterogeneity = 0.002, p < 0.001; Fig. 2a, upper part). The overall tumor control in series including patients with prior surgery was 87.7% (range 84.6–90.9%, I2 = 35.71%, p heterogeneity = 0.1, p < 0.001; Fig. 2a, middle part). The overall tumor control in series not including patients with prior surgery was 93.7% (range 91.9–95.4%, I2 = 0%, p heterogeneity = 0.4, p < 0.001; Fig. 2a, lower part).
Tumor stability
The overall tumor stability in all series was 29.7% (range, 17.7–41.7%, I2 = 96.56%, p heterogeneity < 0.001, p < 0.001; Fig. 2b, upper part). The overall tumor stability in series including patients with prior surgery was 31.2% (range 18.7–43.8%, I2 = 94.33%, p heterogeneity < 0.001, p < 0.001; Fig. 2b, middle part). The overall tumor stability in series not including patients with prior surgery was 23.8% (I2 = 98.75%, p heterogeneity < 0.001, p = 0.16; Fig. 2b, lower part).
Tumor reduction
The overall tumor reduction in all series was observed in 57.0% (range 44.4–69.6%, I2 = 95.43%, p heterogeneity < 0.001, p < 0.001; Fig. 2c, upper part). The overall tumor reduction in series including patients with prior surgery was 55.1% (range 37.8–72.3%, I2 = 96.16%, p heterogeneity < 0.001, p < 0.001; Fig. 2c, middle part). The overall tumor reduction in series not including patients with prior surgery was 64.0% (40.9–87%, I2 = 91.0%, p heterogeneity < 0.001, p < 0.001; Fig. 2c, lower part).
Transient-tumor-expansion (pseudoprogression)
TTE was inconsistently reported. Specifically, only 3/19[24] of the included studies described this outcome. One series reported a crude risk of 41% in 26 patients treated with a median time-to-onset of 8 months (range, 6–13)[17]. Regarding dosimetric predictors, in the series of Chung et al.[12] there was a significant correlation between the T2 signal ratio between tumor and brainstem and the duration of tumor swelling.
Post-SRS procedures
Salvage resection
The overall rate of further microsurgical resection in all series was 7.7% (range 5.3–10.1%, I2 = 69.2%, p heterogeneity < 0.001, p < 0.001; Fig. 3, a, upper part). The overall rate of further microsurgical resection in series including patients with prior surgery was 9.6% (range 6.5–12.6%, I2 = 50.63%, p heterogeneity = 0.01, p < 0.001; Fig. 3, a, middle part). The overall rate of further microsurgical resection in series not including patients with prior surgery was 3.3% (range 1.7–4.9%, I2 = 18.37%, p heterogeneity = 0.29, p < 0.001; Fig. 3, a, lower part).
Post-SRS shunting for hydrocephalus
The overall rate of need for shunt in all series was 5.0% (range, 3.2–6.8%, I2 = 60.92%, p heterogeneity < 0.001, p < 0.001; Fig. 3, b, upper part). The overall rate of further shunt placement in series including patients with prior surgery was 4.7% (range 2.7–6.6%, I2 = 36.07%, p heterogeneity = 0.09, p < 0.001; Fig. 3, b, middle part). The overall rate of further shunt placement in series not including patients with prior surgery was 6.4% (range 2–10.7%, I2 = 75.46%, p heterogeneity = 0.001, p < 0.001; Fig. 3, b, lower part).
Further salvage SRS
The overall rate of salvage SRS in all series was 1.0% (range, 0.5–1.4%, I2 = 0%, p heterogeneity = 0.16, p = 0.941; Fig. 3, c, upper part). The overall rate of further salvage SRS in series including patients with prior surgery was 2.6% (I2 = 41.68%, p heterogeneity = 0.18; Fig. 3, c, middle part). The overall rate of further salvage SRS in series not including patients with prior surgery was 1.0% (range 0.3–1.7%, I2 = 0%, p heterogeneity = 0.83, p = 0.004; Fig. 3, c, lower part).
Cranial nerve toxicities and hearing preservation
The overall rate of facial palsy in all series was 2.3% (range 1.2–3.4%, I2 = 54.47%, p heterogeneity = 0.003, p < 0.001; Fig. 4, a, upper part). The overall rate of new-onset facial palsy in series including patients with prior surgery was 1.3% (range 0.3–2.3%, I2 = 28.00%, p heterogeneity = 0.16, p = 0.01; Fig. 4, a, middle part). The overall rate of facial palsy in series not including patients with prior surgery was 3.4% (range 2.2–4.6%, I2 = 0%, p heterogeneity = 0.52, p < 0.001; Fig. 4, a, lower part).
The overall rate of hearing preservation in all series was 37.9% (range 21.6–54.3%, I2 = 95.96%, p heterogeneity < 0.001, p < 0.001; Fig. 4, b, upper part). The overall rate of hearing preservation in series including patients with prior surgery was 34.2% (range 24.3–44.1%, I2 = 61.5%, p heterogeneity = 0.01, p < 0.001; Fig. 4, b, middle part). The overall rate of hearing preservation in series not including patients with prior surgery was 40.4% (7.0–73.9%, I2 = 98.39%, p heterogeneity < 0.001, p = 0.06; Fig. 4, b, lower part).Tables 2, 3 and 4 summarizes the outcomes.
Discussion
Our systematic review suggests that single fraction SRS could be used for VS ≥ 2.5 cm in maximum diameter, and/or Koos Grade IV, as either the primary treatment modality or for post-operative residual/recurrent tumor. However, we acknowledge that the Koos grade IV tumor definition varies significantly across studies and the minority (6 studies out of 19) of the published literature was specific to upfront treatment.
The overall probability of tumor control (both stability and decrease in volume) and tumor reduction in all series versus those series without prior surgery were 89% versus 93.7%, and 57% versus 64%, respectively. Several of the included series in this meta-analyses identified individual parameters associated with local failure. More specifically, Hasegawa et al. [13] suggested that a high-risk group for lower tumor control included patients with middle cerebellar peduncle compression of ≥ 9.8 mm and ≤ 48 years of age. Tumor control was also higher when prescribing a marginal dose of greater than 12 Gy as compared with less than 12 Gy [19] and for those smaller volumes [18]. Previous microsurgery, tumor volumes exceeding 10 mL, Koos grade IV [8], tumor volume more than 15 mL [14] and progression of residual disease preceding SRS [21] were also factors resulting in lower local control rates. These findings informed the ISRS recommendations as summarized in Table 5.
The rates of salvage microsurgical resection, need for shunt, and additional SRS in all series versus those series with no prior surgery were 9.6% vs 3.3%, and 4.7% vs 6.4% and 1% vs 0.9%, respectively. The rates of facial palsy and hearing preservation in all series versus those series with no prior surgery were 2.3% vs 3.4% and 37.9% vs 40.4%, respectively. Preservation of the facial nerve function was associated with smaller tumor volumes (less than 10 mL) and lower margin dose (≤ 13 Gy) [19]. Deterioration of facial nerve function was associated with a prescription dose of ≥ 13 Gy and early TTE [28]. Hearing preservation was higher in patients with good pre-therapeutic levels of hearing (Gardner Robertson class 1), younger age, and a dose of less than 4 Gy to the cochlea/modiolus (the mean dose/point dose of less than 4 Gy to the cochlea/modiolus being already reported in the literature during the past 15 years and in the overall context of hearing preservation after SRS for VS [29, 30]). Cranial nerve complication rates were suggested in few of reviewed studies to be greater in those VS with cystic components vs solid [11]. Trigeminal neuropathy was rare and usually transient.
A particular entity that would deserve further analysis, although limited data exist on such topic, is related to previously irradiated large partially cystic VSs that will potentially develop symptomatic mass effect from fluid-dynamic cyst enlargement [31, 32], without definite neoplastic growth of the solid part. In such patients, microsurgical exploration for cyst fenestration/drainage without the need for further resection of the already treated tumor cells can be a valuable option, in the absence of tumor growth of the solid part. Such surgical option could reveal much safer and with less morbidity, in the absence of planned subtotal resection of the solid, non-growing part.
In the present review, the overall need for shunt for large VSs treated with upfront SRS in series without prior surgery was 6.4%, which is much higher as compared to smaller tumors. Previous series have suggested the need for shunt after SRS after a median time of 15.5 months (range 1.8–37.8) [33]. Hydrocephalus after radiosurgery may thus co-occur with a temporary tumor volume change after radiation and there is a crucial need for careful ongoing clinical and imaging follow-up [33]. Other authors suggested that large tumor size, ring enhancement patterns and high protein level of CSF should be carefully observed during follow-up course [34]. Thus, using programmable/adjustable MR-compatible ventriculo-peritoneal shunts in time might prevent devastating consequences due to increased intracranial pressure and a risk of sudden neurological decline.
Of the 1723 cases in this meta-analysis, four tumor-related deaths were observed. Two were secondary to developed malignant transformation, which accounts for 0.12% of the sample. This low risk is consistent with the literature including a recent meta-analysis [35]. The other two deaths were related to a refractory VS which relapsed 78 months from the time of SRS and the second due to tumor-related subarachnoid hemorrhage (Hasegawa et al.14). Moreover, TTE was inconsistently reported and should be better detailed by further studies on the same topic. Such TTE might be, in some cases, accompanied by acute and subacute radiation effects, which are in vast majority of cases transient [36].
With respect to fractionated radiation, there are as yet limited data with regards to the use of hypofractionnated SRS for large VS [13, 37]. However, there are 6 non-randomized trials [38,39,40,41,42,43] comparing single fraction SRS with fractionated stereotactic radiotherapy (FSRT). There has yet to be significant differences in 5-year tumor control rates between the two techniques to make any firm recommendations. A recent systematic review compared SRS versus FSRT for tumor control in VSs [44]. The authors suggested that the progression-free survival rates were 92–100% for both treatment options, while the risk of facial and trigeminal nerve deterioration was less for patients treated with SRS [44]. It has been also acknowledged that there is a lack of high-quality studies comparing radiation therapy alternatives for patients with VSs [44]. We would still support fractionated stereotactic radiotherapy for large VS given the established practice as a standard of care, and experience in other benign tumors with favorable control rates [45, 46]. However, there is a need for a randomized or prospectively controlled trial comparing single fraction SRS and FSRT in VS, especially in the context of clarifying if functional outcome would be better with single fraction SRS.
The main limitation of the present meta-analysis was the inability to reliably separate outcomes between upfront vs salvage cohorts, and this added complexity to this analyses. We acknowledge that those studies including patients with prior surgery also included cases with upfront SRS, which can contribute to added bias. The definition of large tumors was also extremely heterogeneous. In particular, for those treated in the post-operative residual or recurrent setting. A limited number of series included “staged-volume” SRS strategies, which might have also influenced local control. The same applies to the cystic tumors, which influence the overall results in terms of local control, and in some series they account for as high as 58% of the included cases [24]. However, the results for cystic tumors have not been separately reported in individual series, although it is now well acknowledged that they respond best to SRS as compared to the solid ones [31]. Additionally, there was a lack of uniformity with regards to the follow-up periods, to which ads variations depending on studies to the long-term and even the short-term follow up. Tumor diameters were inconsistently reported. There was also a lack of reported actuarial outcomes, which are different from the crude rates reported in the studies. There were also several different nuances concerning further neurosurgical interventions, considered as adverse events and not counted as treatment failures. Particularly, the surgical management of hydrocephalus was heterogeneous, including ventriculo-peritoneal shunt, ventriculostomy, Ommaya placement or further cyst puncture and timing of further SRS and additional surgical interventions were also extremely variable, as well as for further surgical interventions.
Recommendations
Ideal candidates for SRS in patients with a VSs of a maximum diameter ≥ 2.5 cm and/or classified as Koos Grade IV are those without symptomatic mass effect, without disabling symptoms, with pre-SRS serviceable hearing, and with comorbidities that make resection more risky or those who wish to avoid a resection (class C evidence). Based on the analyzed data, we conclude that local tumor control is optimal when prescribing a marginal dose between 11 and 13 Gy (class C evidence). Lower rates of tumor control were associated with prior surgical resection, volumes exceeding 10 mL, large Koos grade IV, progression of residual VS prior to SRS and middle cerebellar peduncle compression (class C evidence). Better facial nerve preservation was observed when treating tumor volumes less than 10 mL, non-cystic VS, and when thw marginal dose lower is than 13 Gy (class C evidence). Better hearing preservation rates were associated with younger patients (age less than 60 years), better initial hearing level (Gardner-Robertson 1) and a cochlear dose of less than 4 Gy (class C evidence). The ISRS recommendations are summarized in Table 5.
Conclusion
Although large VS are considered a classical indication for microsurgical resection, upfront single fraction SRS might be useful in select patients (class C evidence). When analyzing data from those series with no prior surgery vs those with prior surgery, higher rates of tumor control, further tumor reduction, lower rates of further intervention (microsurgical resection, shunt, SRS), higher rates of “de novo” facial palsy (although overall low) and higher hearing preservation rates were observed (class C evidence).
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request. They are however described in details in tables and figures.
References
Tsao MN, Sahgal A, Xu W, De Salles A, Hayashi M, Levivier M, Ma L, Martinez R, Regis J, Ryu S, Slotman BJ, Paddick I (2017) Stereotactic radiosurgery for vestibular schwannoma: International Stereotactic Radiosurgery Society (ISRS) Practice Guideline. J Radiosurg SBRT 5:5–24
Regis J, Carron R, Park MC, Soumare O, Delsanti C, Thomassin JM, Roche PH (2010) Wait-and-see strategy compared with proactive Gamma Knife surgery in patients with intracanalicular vestibular schwannomas. J Neurosurg 113(Suppl):105–111. https://doi.org/10.3171/2010.8.GKS101058
Samii M, Gerganov VM, Samii A (2010) Functional outcome after complete surgical removal of giant vestibular schwannomas. J Neurosurg 112:860–867. https://doi.org/10.3171/2009.7.JNS0989
Samii M, Metwali H, Gerganov V (2016) Microsurgical management of vestibular schwannoma after failed previous surgery. J Neurosurg 125:1198–1203. https://doi.org/10.3171/2015.8.JNS151350
Carlson ML, Link MJ (2021) Vestibular Schwannomas. N Engl J Med 384:1335–1348. https://doi.org/10.1056/NEJMra2020394
Lefranc M, Da Roz LM, Balossier A, Thomassin JM, Roche PH, Regis J (2018) Place of gamma knife stereotactic radiosurgery in grade 4 vestibular schwannoma based on case series of 86 patients with long-term follow-up. World Neurosurg 114:e1192–e1198. https://doi.org/10.1016/j.wneu.2018.03.175
van de Langenberg R, Hanssens PE, van Overbeeke JJ, Verheul JB, Nelemans PJ, de Bondt BJ, Stokroos RJ (2011) Management of large vestibular schwannoma. Part I. Planned subtotal resection followed by Gamma Knife surgery: radiological and clinical aspects. J Neurosurg 115:875–884. https://doi.org/10.3171/2011.6.JNS101958
Yang HC, Kano H, Awan NR, Lunsford LD, Niranjan A, Flickinger JC, Novotny J Jr, Bhatnagar JP, Kondziolka D (2011) Gamma Knife radiosurgery for larger-volume vestibular schwannomas. Clinical article. J Neurosurg 114:801–807. https://doi.org/10.3171/2010.8.JNS10674
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol 62:e1-34. https://doi.org/10.1016/j.jclinepi.2009.06.006
Koos WT, Day JD, Matula C, Levy DI (1998) Neurotopographic considerations in the microsurgical treatment of small acoustic neurinomas. J Neurosurg 88:506–512. https://doi.org/10.3171/jns.1998.88.3.0506
Bailo M, Boari N, Franzin A, Gagliardi F, Spina A, Del Vecchio A, Gemma M, Bolognesi A, Mortini P (2016) Gamma knife radiosurgery as primary treatment for large vestibular schwannomas: clinical results at long-term follow-up in a series of 59 patients. World Neurosurg 95:487–501. https://doi.org/10.1016/j.wneu.2016.07.117
Chung WY, Pan DH, Lee CC, Wu HM, Liu KD, Yen YS, Guo WY, Shiau CY, Shih YH (2010) Large vestibular schwannomas treated by Gamma Knife surgery: long-term outcomes. J Neurosurg 113(Suppl):112–121. https://doi.org/10.3171/2010.8.GKS10954
Hasegawa T, Kato T, Naito T, Tanei T, Ishii K, Tsukamoto E, Okada K, Ito R, Kouketsu Y (2021) Predictors of long-term tumor control after stereotactic radiosurgery for Koos grade 4 vestibular schwannomas. J Neurooncol 151:145–156. https://doi.org/10.1007/s11060-020-03622-5
Huang CW, Tu HT, Chuang CY, Chang CS, Chou HH, Lee MT, Huang CF (2018) Gamma Knife radiosurgery for large vestibular schwannomas greater than 3 cm in diameter. J Neurosurg 128:1380–1387. https://doi.org/10.3171/2016.12.JNS161530
Huo M, Foley H, Pinkham M, Shanker M, Bernard A, Jenkins M, Olson S, Hall B, Watkins T, Jones C, Foote M (2020) Stereotactic radiotherapy for large vestibular schwannomas: Volume change following single fraction versus hypofractionated approaches. J Radiosurg SBRT 7:11–17
Inoue HK (2005) Low-dose radiosurgery for large vestibular schwannomas: long-term results of functional preservation. J Neurosurg 102(Suppl):111–113
Iorio-Morin C, AlSubaie F, Mathieu D (2016) Safety and efficacy of gamma knife radiosurgery for the management of Koos grade 4 vestibular schwannomas. Neurosurgery 78:521–530. https://doi.org/10.1227/NEU.0000000000001154
Mezey G, Cahill J, Rowe JG, Yianni J, Bhattacharyya D, Walton L, Rodgers J, Radatz MWR (2020) A retrospective analysis of the role of single-session gamma knife stereotactic radiosurgery in sporadic vestibular schwannomas with tumor volumes greater than 10 cm3: is it worth stretching the boundaries? Stereotact Funct Neurosurg 98:85–94. https://doi.org/10.1159/000504857
Ogino A, Lunsford LD, Long H, Johnson S, Faramand A, Niranjan A, Flickinger JC, Kano H (2021) Stereotactic radiosurgery as the primary management for patients with Koos grade IV vestibular schwannomas. J Neurosurg. https://doi.org/10.3171/2020.8.JNS201832
Pikis S, Mantziaris G, Kormath Anand R, Nabeel AM, Sheehan D, Sheehan K, Reda WA, Tawadros SR, Abdelkarim K, El-Shehaby AMN, Emad Eldin R, Peker S, Samanci Y, Kaisman-Elbaz T, Speckter H, Hernandez W, Isidor J, Tripathi M, Madan R, Zacharia BE, Daggubati LC, Martinez Moreno N, Martinez Alvarez R, Langlois AM, Mathieu D, Deibert CP, Sudhakar VR, Cifarelli CP, Arteaga Icaza D, Cifarelli DT, Wei Z, Niranjan A, Barnett GH, Lunsford LD, Bowden GN, Sheehan JP (2022) Stereotactic radiosurgery for Koos grade IV vestibular schwannoma: a multi-institutional study. J Neurosurg. https://doi.org/10.3171/2022.4.JNS22203
Stastna D, Urgosik D, Chytka T, Liscak R (2021) Large vestibular schwannomas: long-term outcomes after stereotactic radiosurgery. Neuro Endocrinol Lett 41:329–338
Umekawa M, Shinya Y, Hasegawa H, Kawashima M, Shin M, Katano A, Minamitani M, Kashio A, Kondo K, Saito N (2022) Stereotactic radiosurgery ensures an effective and safe long-term control of Koos grade IV vestibular schwannomas: a single-center, retrospective, cohort study. J Neurooncol 159:201–209. https://doi.org/10.1007/s11060-022-04058-9
van de Langenberg R, Hanssens PE, Verheul JB, van Overbeeke JJ, Nelemans PJ, Dohmen AJ, de Bondt BJ, Stokroos RJ (2011) Management of large vestibular schwannoma. Part II. Primary Gamma Knife surgery: radiological and clinical aspects. J Neurosurg 115:885–893. https://doi.org/10.3171/2011.6.JNS101963
Watanabe S, Yamamoto M, Kawabe T, Koiso T, Aiyama H, Kasuya H, Barfod BE (2019) Long-term follow-up results of stereotactic radiosurgery for vestibular schwannomas larger than 8 cc. Acta Neurochir 161:1457–1465. https://doi.org/10.1007/s00701-019-03951-z
Williams BJ, Xu Z, Salvetti DJ, McNeill IT, Larner J, Sheehan JP (2013) Gamma Knife surgery for large vestibular schwannomas: a single-center retrospective case-matched comparison assessing the effect of lesion size. J Neurosurg 119:463–471. https://doi.org/10.3171/2013.4.JNS122195
Zeiler FA, Bigder M, Kaufmann A, McDonald PJ, Fewer D, Butler J, Schroeder G, West M (2013) Gamma knife radiosurgery for large vestibular schwannomas: a Canadian experience. Can J Neurolog Sci 40:342–347. https://doi.org/10.1017/s0317167100014281
Milligan BD, Pollock BE, Foote RL, Link MJ (2012) Long-term tumor control and cranial nerve outcomes following gamma knife surgery for larger-volume vestibular schwannomas. J Neurosurg 116:598–604. https://doi.org/10.3171/2011.11.JNS11811
Pikis S, Mantziaris G, Anand RK, Nabeel AM, Sheehan D, Sheehan K, Reda WA, Tawadros SR, Abdelkarim K, El-Shehaby AMN, Eldin RE, Peker S, Samanci Y, Kaisman-Elbaz T, Speckter H, Hernández W, Isidor J, Tripathi M, Madan R, Zacharia BE, Daggubati LC, Martínez Moreno N, Martínez Álvarez R, Langlois A-M, Mathieu D, Deibert C, Sudhakar V, Cifarelli C, Arteaga Icaza D, Cifarelli D, Wei Z, Niranjan A, Barnett G, Lunsford D, Bowden G, Sheehan J (2022) Stereotactic radiosurgery for Koos grade IV vestibular schwannoma: a multi-institutional study. J Neurosurg. https://doi.org/10.3171/2022.4.JNS22203
Massager N, Nissim O, Delbrouck C, Delpierre I, Devriendt D, Desmedt F, Wikler D, Brotchi J, Levivier M (2013) Irradiation of cochlear structures during vestibular schwannoma radiosurgery and associated hearing outcome. J Neurosurg 119(Suppl):733–739
Tamura M, Carron R, Yomo S, Arkha Y, Muraciolle X, Porcheron D, Thomassin JM, Roche PH, Regis J (2009) Hearing preservation after gamma knife radiosurgery for vestibular schwannomas presenting with high-level hearing. Neurosurgery 64:289–296. https://doi.org/10.1227/01.NEU.0000338256.87936.7C
Bowden G, Cavaleri J, Monaco E III, Niranjan A, Flickinger J, Lunsford LD (2017) Cystic vestibular schwannomas respond best to radiosurgery. Neurosurgery 81:490–497. https://doi.org/10.1093/neuros/nyx027
Shuto T, Matsunaga S (2016) Two cases of cystic enlargement of vestibular schwannoma as a late complication following gamma knife surgery. J Clin Neurosci 33:239–241. https://doi.org/10.1016/j.jocn.2016.05.022
Lee SH, Seol HJ, Kong DS, Nam DH, Park K, Kim JH, Lee JI (2012) Risk factors and tumor response associated with hydrocephalus after gamma knife radiosurgery for vestibular schwannoma. Acta Neurochir 154:1679–1684. https://doi.org/10.1007/s00701-012-1350-0
Shimizu Y, Miyamori T, Yamano J (2019) Hydrocephalus after gamma knife radiosurgery for schwannoma. Asian journal of neurosurgery 14:487–490. https://doi.org/10.4103/ajns.AJNS_278_18
Wolf A, Naylor K, Tam M, Habibi A, Novotny J, Liscak R, Martinez-Moreno N, Martinez-Alvarez R, Sisterson N, Golfinos JG, Silverman J, Kano H, Sheehan J, Lunsford LD, Kondziolka D (2019) Risk of radiation-associated intracranial malignancy after stereotactic radiosurgery: a retrospective, multicentre, cohort study. Lancet Oncol 20:159–164. https://doi.org/10.1016/S1470-2045(18)30659-4
Tuleasca C, George M, Faouzi M, Schiappacasse L, Leroy HA, Zeverino M, Daniel RT, Maire R, Levivier M (2016) Acute clinical adverse radiation effects after Gamma Knife surgery for vestibular schwannomas. J Neurosurg 125:73–82. https://doi.org/10.3171/2016.7.GKS161496
Teo M, Zhang M, Li A, Thompson PA, Tayag AT, Wallach J, Gibbs IC, Soltys SG, Hancock SL, Chang SD (2016) The outcome of hypofractionated stereotactic radiosurgery for large vestibular schwannomas. World Neurosurg 93:398–409. https://doi.org/10.1016/j.wneu.2016.06.080
Anderson BM, Khuntia D, Bentzen SM, Geye HM, Hayes LL, Kuo JS, Baskaya MK, Badie B, Basavatia A, Pyle GM, Tome WA, Mehta MP (2014) Single institution experience treating 104 vestibular schwannomas with fractionated stereotactic radiation therapy or stereotactic radiosurgery. J Neurooncol 116:187–193. https://doi.org/10.1007/s11060-013-1282-4
Andrews DW, Suarez O, Goldman HW, Downes MB, Bednarz G, Corn BW, Werner-Wasik M, Rosenstock J, Curran WJ Jr (2001) Stereotactic radiosurgery and fractionated stereotactic radiotherapy for the treatment of acoustic schwannomas: comparative observations of 125 patients treated at one institution. Int J Radiat Oncol Biol Phys 50:1265–1278. https://doi.org/10.1016/s0360-3016(01)01559-0
Collen C, Ampe B, Gevaert T, Moens M, Linthout N, De Ridder M, Verellen D, D’Haens J, Storme G (2011) Single fraction versus fractionated linac-based stereotactic radiotherapy for vestibular schwannoma: a single-institution experience. Int J Radiat Oncol Biol Phys 81:e503-509. https://doi.org/10.1016/j.ijrobp.2011.04.066
Combs SE, Engelhard C, Kopp C, Wiedenmann N, Schramm O, Prokic V, Debus J, Molls M, Grosu AL (2015) Long-term outcome after highly advanced single-dose or fractionated radiotherapy in patients with vestibular schwannomas - pooled results from 3 large German centers. Radiother Oncol 114:378–383. https://doi.org/10.1016/j.radonc.2015.01.011
Kopp C, Fauser C, Muller A, Astner ST, Jacob V, Lumenta C, Meyer B, Tonn JC, Molls M, Grosu AL (2011) Stereotactic fractionated radiotherapy and LINAC radiosurgery in the treatment of vestibular schwannoma-report about both stereotactic methods from a single institution. Int J Radiat Oncol Biol Phys 80:1485–1491. https://doi.org/10.1016/j.ijrobp.2010.04.057
Meijer OW, Vandertop WP, Baayen JC, Slotman BJ (2003) Single-fraction vs fractionated linac-based stereotactic radiosurgery for vestibular schwannoma: a single-institution study. Int J Radiat Oncol Biol Phys 56:1390–1396. https://doi.org/10.1016/s0360-3016(03)00444-9
Persson O, Bartek J Jr, Shalom NB, Wangerid T, Jakola AS, Forander P (2017) Stereotactic radiosurgery vs fractionated radiotherapy for tumor control in vestibular schwannoma patients: a systematic review. Acta Neurochir 159:1013–1021. https://doi.org/10.1007/s00701-017-3164-6
Pinzi V, Marchetti M, Viola A, Tramacere I, Cane I, Iezzoni C, Fariselli L (2022) Hypofractionated radiosurgery for large or in critical-site intracranial meningioma: results of a phase 2 prospective study. Int J Radiat Oncol Biol Phys. https://doi.org/10.1016/j.ijrobp.2022.08.064
Nguyen T, Duong C, Sheppard JP, Lee SJ, Kishan AU, Lee P, Tenn S, Chin R, Kaprealian TB, Yang I (2018) Hypo-fractionated stereotactic radiotherapy of five fractions with linear accelerator for vestibular schwannomas: A systematic review and meta-analysis. Clin Neurol Neurosurg 166:116–123. https://doi.org/10.1016/j.clineuro.2018.01.005
Acknowledgements
N/A
Funding
Open access funding provided by University of Lausanne. None.
Author information
Authors and Affiliations
Contributions
First draft of the manuscript: CT; critically revising the first draft: RK, AS, SY, ML; critically revising the second draft: all authors; statistical analysis: CT; materiel and technical support: CT; revised submitted version: all authors.
Corresponding author
Ethics declarations
Competing interests
None.
Ethical approval
N/A.
Consent to participate
N/A.
Consent to publish
N/A.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Disclaimer: These guidelines should not be considered inclusive of all methods of care or exclusive of other methods or care reasonably directed to obtain similar results. The physician must make the ultimate judgment depending on characteristics and circumstances of individual patients. Adherence to this guideline will not ensure successful treatment in every situation. The authors of this guideline and the International Society of Stereotactic Radiosurgery assume no liability for the information, conclusions, and recommendations contained in this report.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tuleasca, C., Kotecha, R., Sahgal, A. et al. Single-fraction radiosurgery outcomes for large vestibular schwannomas in the upfront or post-surgical setting: a systematic review and International Stereotactic Radiosurgery Society (ISRS) Practice Guidelines. J Neurooncol 165, 1–20 (2023). https://doi.org/10.1007/s11060-023-04455-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-023-04455-8