Abstract
Purpose
Exercise proved to reduce cancer-related symptoms and prolong survival in some cancer types. However, brain tumor patients are often advised against strenuous exercise. Here, we summarize our experience with a submaximal exercise program for glioma patients: ActiNO (Active in Neuro-Oncology).
Methods
Glioma patients were invited to participate in the program. Since 2011, a sports scientist individualized two one-hour sessions per week adapted to the patients’ symptoms. One session consisted of bicycle ergometry (average workload: 75% of maximum heart rate), the other of whole-body resistance training. Both sessions were further complimented by coordinative elements. Cardiorespiratory fitness was assessed using the ”Physical Work Capacity” procedure. Patients were followed up regularly to assess adherence to the program and disease activity.
Results
Until December 2019, 45 glioma patients, median-aged 49 years (IQR 42–59), were included in the analysis. Most patients suffered from glioblastoma (58%), followed by diffuse lower-grade astrocytoma (29%). In overall 1828 training sessions, two minor epileptic events occurred (1 speech arrest; 1 focal seizure). During fitness assessment, all patients achieved at least 75% of their age-adjusted maximum heart rate. Peak workload averaged 172 W (95% CI 156–187). Median survival of participating glioblastoma patients was 24.1 months (95% CI 8.6–39.5).
Conclusion
This supervised training program with submaximal exertion was feasible and safe in glioma regardless of WHO grading. Based on these experiences, we initiated a prospective multicenter study to objectify improvements in physical performance and quality of life in patients with glioblastoma.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Despite multimodal therapies, malignant gliomas still have a poor prognosis [1]. Many glioma patients suffer from disease- or therapy-induced neurocognitive, functional, and emotional deficits, which significantly limit quality of life [2]. The main goal of all treatment efforts is therefore a prolongation of life at the highest possible quality. To reach that goal, supportive therapies are well perceived [3].
Out of many suitable supportive therapies, sportive activities and physical training for cancer patients are considered ideal for cancer patients at large. In almost 700 clinical trials with more than 50,000 cancer patients examined at different treatment time points, many positive effects of exercise, especially regarding side effect management including fatigue, polyneuropathies, psychological distress, and physical constraints have been demonstrated [3]. However, most intervention studies were conducted in patients with breast, prostate, and colon cancer. In these entities, physical training is safe, feasible, and potentially beneficial regarding patients’ physical, emotional, and cognitive well-being [4,5,6].
In contrast, little is known about the benefit to glioma patients [7]. In a systematic review by Sandler et al. comprising 15 studies, it was shown that the majority of patients are not sufficiently physically active after being diagnosed with a brain tumor, although more active patients showed improved quality of life as well as less severe symptom burden [8]. Given that unmet need, a personal training program for brain tumor patients was initiated at our institution. Here, we describe the setup for an intensive supervised exercise program, demonstrate its feasibility and safety, and analyze outcomes.
Conceptual development of the exercise program
At start, the personal training program was rather unsystematically and was shaped by the participants’ individual wishes and ideas. General recommendations for cancer patients at that time, i.e., published by the American College of Sports Medicine, were considered [9]. As they focused on breast, prostate, hematologic, colorectal, and gynecologic cancers, but not on brain tumor patients, it was decided that the exercise program for multimodal treated glioma patients should be individualized. Regarding training modalities and intensity, it should be optimally conducted twice per week for 1 hour and should contain both strength and endurance elements in order to be able to achieve noticeable increases in performance. Furthermore, a special focus was placed on the integration of coordinative elements. In general, exercise tasks were adapted to the patients’ respective symptoms. If possible, exercise levels were enhanced over time. Heart rate was monitored throughout the entire session (via Polar M430 incl. HR sensor H7/9, Kempele, Finland).
As the number of patients increased, the training became more systematic. We concluded that training should ideally be conducted on a 1:1-basis. Usually, each session was divided into approximately 50 min of either resistance or endurance training and completed by 10 min of coordination training (Fig. 1).
Endurance training
Endurance training is performed on a bicycle ergometer and divided into 4 parts. Due to having 5 fix points to the anti-tilt bicycle ergometer (feet, hands and bottom), even patients with balance disorders feel safe and can be loaded properly. Adjustments to workload are controlled by heart rate. Continuous speed of rotation is 70–75 rpm. After a 10-min warm-up, patients achieve 60–65% of their individual maximum heart rate (HRmax). In the next 12-min-part, 3 intervals of coordinative elements using 1–2 kg-dumbbells are integrated, workload remains constant (Suppl. Figure S1). This way, fix points are reduced to 3 (feet and bottom) and the patients’ trunk muscles are activated to compensate for any imbalances. Heart rate meanwhile increases up to 75% of HRmax. This is followed by five 2-min-intervals (1-min resting interval each), increasing in intensity (last interval at about 90% of HRmax). The training concludes with a 6-min cool-down. An average workload of 75% of HRmax throughout the session is targeted (Fig. 2).
Illustration of a typical endurance training sequence (part 1 to part 4). Numbers on abscissa indicate time in minutes, ordinate reflects heart rate per minute, measured with Polar M430 and HR sensor H7/9. After warm-up, the patient is exposed to different interventions in addition to ergometric cycling (part 2). To visualize the coordinative elements during part 2, a series of dumbbell movements is shown in Supplementary Figure S1. To achieve submaximal loading, a high-intensity interval training (part 3) is performed prior to the cool-down phase (part 4). Upon completion of endurance training, approx. 10 min of coordination training (i.e. Hoop pyramid (Fig S2) or arm skill training (Fig. S3)) sums up to 60 min of training.
Resistance training
Progressive whole-body resistance training is based on up to 12 separate, but standardized exercise tasks (leg press, knee extension + flexion, adductors, abductors, butterfly + reverse, chest press, biceps + triceps curls, latissimus pulldown, abdominal machine). 3 sets of 21 repetitions each are performed. Rate of perceived exertion (RPE, scale 1–20) is at 14 when entering the program. As training progresses, intensity reaches RPE 17–20. Rests between sets last 30–60 s. A special feature of the exercise execution is a change in movement amplitude in the course of 21 repetitions. The first 7 repetitions are performed with maximum amplitude, the following 7 with half amplitude and the last 7 are small final contractions. This method is based on electromyography analyses according to Boeckh-Behrens & Buskies and aims at maximal muscle activation [10]. Execution is performed rather quickly, so that each series lasts 30–40 s. Total time under tension is 90–120 s.
Coordination training
Coordination training consists of either arm skill training or exercises including agility hoops arranged as a pyramid. Arm skill training is based on a complex choreography including bending, stretching, and rotation tasks to both improve arm control and challenge the brain. During hoop training, patients have to cross a hoop pyramid by following a given sequence of steps, increasing in complexity (e.g., starting with 2 contacts per hoop). Further explanations on coordination training can be taken from the Supplement.
Methods
Study design and setting
This is a retrospective observational study in a single tertiary academic center setting. As part of the psycho-oncological consultation after surgical resection, patients at the brain tumor center of the University Hospital Münster, who were found to be suitable (interested in exercise, no severe neurologic or cognitive deficits precluding patient participation, no refractory epileptic seizures (> 3 focal seizures per day or > 1 generalized seizure within 3 days of evaluation), ECOG 0–1, age ≥ 18), were offered participation in a free personal training program. Data were extracted from the patients’ charts. Beside notion of standard baseline characteristics, adverse events during training were rated according to the Common Terminology Criteria for Adverse Events (CTCAE), version 5.0. For reporting the “Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Guidelines” were adapted. Ethical approval was obtained from the regional ethical committee (2020-296-f-S).
Data sources and measurements
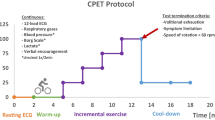
We analyze all glioma patients that took part in the program described above from its initiation in 2011 until 2019. To assess cardiorespiratory fitness of all patients at the program’s start, an incremental, physician-monitored exercise test with continuous heart rate monitoring was performed according to guidelines published by the WHO [11]. Maximum loading was targeted to achieve the highest possible diagnostic accuracy. All tests were performed on a cycle ergometer (Ergo-Fit 3000 Cycle med, Pirmasens, Germany). Participants began cycling at 25 W. Workloads were then increased by 25 W every 2 min until volitional exhaustion. Patients were asked to maintain a speed of 70 rpm. At the end of each workload, rating of perceived exertion was evaluated using the Borg scale.
Physical fitness was determined based on heart rate data using the “Physical Work Capacity” (PWC) procedure [12, 13]. Pulse-related power in watts at 75% of age-adjusted HRmax according to Tanaka et al. [14] was calculated using linear interpolation [15]. Subsequently, the calculated PWC values were divided by the patients’ body weight to determine the achieved watts per kilogram of body weight (W/kg BW). PWC performance served as an orientation for the sports scientist to create the participants’ individual training protocol. Provided that organizational and personnel resources were available, a second cardiorespiratory exercise test was conducted to detect changes in physical fitness.
To exclude negative effects on overall survival due to intensive exercise, a survival analysis of all participating glioblastoma patients (WHO grade 4, n = 26) was introduced. For comparison, the basis for the control group were all consecutive adult patients who underwent surgery for glioblastoma at the institution between January 2011 and December 2019. Of these, the control group was carefully selected based on comparable criteria, including nutrition status (BMI), age, and received adjuvant therapies, to minimize potential selection bias. As an additional supplement to this analysis, a matched-pair analysis with all IDH-wildtype glioblastoma patients was conducted. Matching was performed based on age (with a tolerance of plus/minus 3 years), gender (exact match required), having active or previous adjuvant treatment (exact match required), MGMT status (O-6-methylguanine-DNA methyltransferase, exact match required), and BMI (with a tolerance of plus/minus 3 kg/m²).
Statistical methods
Standard descriptive analyses were performed. Absolute and relative frequencies were used for categorical variables. Means and standard deviations (SD) were shown for normally distributed data and medians and ranges or interquartile ranges (IQRs) for data not normally distributed. Data from PWC tests were tested for normal distribution using the Shapiro-Wilk test. Mean comparison between the two test time points was then performed using either dependent samples t-test (if normally distributed) or Mann-Whitney U test (not normally distributed). Overall survival was estimated using the Kaplan-Meier method and assessed for statistical significance with the log-rank test. Data were analyzed using GraphPad Prism Version 9.0 (GraphPad Software, San Diego, CA, USA) and SPSS Version 29.0 (IBM, Armonk, NY, USA).
Results
Participants
Fourty-five patients (42% female), median-aged 49 years (95% CI 23–76), were included in this individual exercise program of overall 1828 training sessions. Mean BMI was 24 kg/m2 (95% CI 18–33) at the time of training commencement. Most patients suffered from glioblastoma (n = 26, 58%, referring to the WHO classification from 2016 [16]), followed by diffuse lower-grade astrocytoma (29%). The majority received subsequent treatment (Table 1). Except for one patient, all participating glioblastoma patients were undergoing adjuvant treatment at the time of training commencement and the majority of them still received concurrent radiochemotherapy (16/26, 61%).
Cardiorespiratory exercise testing
All patients managed a cardiorespiratory exercise test upon entry. At 75% of their age-adjusted HRmax, they performed an average workload of 1.46 W/kg BW (range 0.5–2.4) prior entering the training program. Female patients performed an average workload of 1.17 W/kg BW (range 0.5-2.0); the corresponding power score for male patients was 1.66 W/kg BW (range 0.7–2.4) (data not shown). During this open, voluntary exercise program, it was possible to perform a second exercise test in 15 patients (6 women). Their mean score was 1.48 W/kg BW at the beginning of the program and increased to 1.65 W/kg BW (p = 0.003), an improvement of 11.5% on average (p < 0.003, intra-individual comparison). Regarding sex, women’s average score increased from 1.25 W/kg BW (range 0.8–1.7) to 1.48 W/kg BW (range 1.2–1.9), and the score for men raised from 1.63 W/kg BW (range 1.2–2.2) to 1.77 W/kg BW (1.2–2.2). In total, eleven patients improved (73%), one patient maintained his performance, and three deteriorated (Fig. 3).
Despite the increase in workload to volitional exhaustion, no complications occurred during incremental exercise testing. Due to weakness of the legs, one patient had to stop at a perceived effort of 15. All patients achieved at least 75% of their age-adjusted heart rate. Peak workload averaged 172 W (range 50–275 W, 95% CI 156–187) and the mean perceived effort at peak was 18.7 (Borg scale, range 15–20, 95% CI 18.3–19.1).
Adverse events during training
During 1828 training sessions, only two self-limiting seizures (focal seizure and speech arrest, both grade 1 according to CTCAE Version 5) occurred (0.1%). Both patients were familiar with the respective type of seizure and training was continued with reduced intensity. No training-related moderate or severe adverse events (grade 2–4) were observed.
Follow-up
The patients accomplished a median of 16 (IQR 7–49) training sessions. Thirty-six (80%) patients quit the training program. The most common reason (44%) to discontinue training was tumor progression/death, followed by infrastructural reasons (i.e., distance between training site and home). Until today, more than 10 years after program initiation, 27 of the 45 (60%) patients have died. Of the 18 survivors, 15 (83%) continue exercising: 9 patients continue training on their own (counseling offered if needed), and further 6 patients exercise under occasional supervision. One patient had to stop exercising after suffering a brainstem stroke 15 month after entering the training program and two patients are lost to follow-up.
Survival analysis for glioblastoma
From 2011 to 2019, 579 glioblastoma patients were surgically treated at the University Hospitals’ Brain Tumor Center. Median survival of all consecutive adult patients was 11.8 month (95% CI 9.1–14.5; data not shown).The median survival of ActiNO participants with glioblastoma (n = 26) was 24.1 months (95% CI 8.6–39.5). To address the positive selection of the ActiNO participants (all of them received adjuvant multimodal therapy including radiochemotherapy with temozolomide [17] or a combination of lumostine and temozolomide [18], their younger age (the oldest ActiNO participant was 76 years), and their better nutrition status (the highest BMI was 32.9 kg/m2), the control cohort should have the same selection criteria (all were treated multimodally with radiochemotherapy, age ≤ 77 years, BMI ≤ 33 kg/m2). Given that, 325 out of 579 patients (56%) met these criteria. The ActiNO group and the control cohort did not significantly differ in terms of age and BMI (p > 0.05, all comparisons). The median survival of this carefully chosen control cohort (n = 325) was 16.0 month (95% CI 14.2–17.9), p < 0.005 compared to the ActiNO group) (Fig. 4A). Due to the solely descriptive character of the study, the large difference in sample size of the two groups, and the potential selection bias of the ActiNO group, further Cox proportional hazard regression analysis was not performed. The survival benefit of the ActiNO group is considered clinically meaningful but hypothesis generating only. To further strengthen this observation, and to raise the evidence that ActiNO-activities definitely did not harm, a matched-pair analysis was conducted (Fig. 4B). Although the survival curves separate quite similarly, the observed difference did not reach statistical significance (p = 0.099) due to the limited sample size. However, a clear trend favoring the ActiNO group is still discernible.
Kaplan Meier plot demonstrates a relationship between overall survival and participating in an individualized, voluntary, intensive exercise program. Figure 4 A displays a median survival of the participating patients with glioblastoma of 24.1 months (95% CI 8.6–39. 5) compared to an age, therapy-, and nutritional-selected comparator mimicking the exercise intervention group. The median survival of comparator group was 16.0 months (95% CI 14.2–17.9), p < 0.005). Figure 4B shows a matched-pair analysis in IDH-wildtype glioblastoma patients (n = 19 in each group); due to the small sample size, statistical power is limited, p = 0.099. Intensive training does not harm, however the conclusion of a survival benefit as a direct consequence of intensive exercise training cannot be drawn due to the retrospective, solely descriptive data analysis.
Discussion
We report on the first series of intensified physical exercise for diffuse glioma patients including glioblastoma. Based on our experience with over 1800 training sessions in a total of 45 patients, several major conclusions can be drawn.
First, submaximal physical training is tolerable and safe in selected patients with diffuse glioma, even in high-grade gliomas, and even under adjuvant therapies. High-intensity training despite various impairments is possible. We saw no falls or other exercise-related injuries. Only two minor focal seizures (0.1% of training sessions) were noted. Seizures did not reoccur during subsequent training and patients were willing and able to continue training immediately. In a systematic review van den Bongard et al. found that exercise does not usually have a negative effect on seizure frequency in epileptics [19]. Furthermore, our experience is consistent with that of 13 studies that demonstrated safety of less intensive exercise programs in brain tumor patients [8].
Second, incremental exercise testing to volitional exhaustion under supervision is a safe and feasible procedure, which has not been demonstrated before. To the best of our knowledge, there is only one study in glioblastoma patients with maximal exercise stress during cardiorespiratory exercise testing. In contrast to our study, some of those patients were unable to achieve maximal cardiovascular function due to fatigue and leg weakness [20].
Third, and in line with other interventional studies in glioma patients [4, 20,21,22,23,24], we were able to demonstrate an improvement in physical performance through a structured exercise program. Although the studies cited are small samples, a clear trend can be seen for both strength parameter and cardiorespiratory fitness. Nevertheless, it should be noted that a certain level of exercise intensity might be necessary to increase physical fitness. In addition, it has been shown that the greatest benefit in quality of life in cancer patients was achieved with exercise programs of at least 120 min of activity per week, for at least 2 month, including a minimum of 15 min of high-intensity training [25]. Appropriate intervention studies testing more intensive versus less intensive training on several outcome parameters (incl. quality of life and physical functioning) in brain tumor patients are warranted. Of note, one of our glioblastoma patients managed to run a marathon and kept improving his performance despite multimodal therapy [26]. This example emphasizes that extreme exercise exposures are feasible for selected glioma patients.
Looking at the high adherence rates to exercise programs for brain tumor patients (ranging from 79 to 100%), motivation and interest in sports are seemingly high [4, 6, 22, 23]. Similar to Capozzi et al. [24], disease progression and infrastructural reasons were the most common causative factors for discontinuation of exercise. Another motivational component is the personal contact with the trainer. A systematic review has shown that supervised training is more effective than unsupervised exercise [27].
The fourth major observation is that high intensive physical exercise does not negatively impact survival in high-grade brain tumor patients. Quite opposite, patients receiving submaximal exercise training showed a trend towards prolonged survival. This has not yet been reported for glioblastoma. In other cancers, higher levels of physical activity are related to positive impacts on survival outcomes [28]. A randomized controlled trial including 242 breast cancer patients showed that exercise improved chemotherapy tolerance [29]. Furthermore, follow-up suggests a more favorable, but non-significant disease-free survival as well as overall survival for both breast cancer exercise groups [30]. In addition, in the observational study by Holmes et al. [31], breast cancer patients being more active had a reduced risk of mortality. Regular physical activity has also been shown to prolong survival in colorectal cancer [32,33,34]. Similarly, there are associations between lower overall mortality and cancer-specific mortality in men with prostate cancer [35].
With regard to the Kaplan-Meier analysis, a harmful effect on overall survival due to maximal physical activity can be excluded in glioblastoma patients. Moreover, despite carefully matching, and a still possible selection bias of the participants in this voluntary program, a trend towards prolonged survival is visible and hypothesis generating. Further studies have to prove possible survival benefits.
The potential mechanism to create a survival benefit for glioma patients with increasing physical fitness may be the building of higher muscle strength and muscle mass. Temporal muscle thickness of newly diagnosed glioblastoma patients at baseline as well as the extent of its loss over time, for instance, are of prognostic relevance [36]. Since temporal muscle thickness is highly correlated with muscle strength [37], it may be of particular interest to counteract general muscle wasting with appropriate interventions. Benefits from physical activity have also been shown in mouse models of glioma [38,39,40]. Tantillo et al., e.g., report on reduced tumor proliferation rates as well as later onset of motor deficits by voluntary physical exercise in glioma-bearing mice [40]. Importantly, in the study of Lemke et al., this prolonged survival through exercise was significant only when combined with temozolomide treatment [39].
The main limitation of our study is its retrospective character and the heterogeneity of tumor types. Potential selection biases reduce the likelihood of generalizability of the results. When comparing with the performance values of a large representative German cohort [41], the glioma patients tested here show a trend towards above average performance values at training commencement. Those patients, athletic and interested in sports, may maintain superior physical fitness even in case of severe disease, resulting in potentially favorable long-term outcome.
Conclusion
Our data demonstrate that intensive exercise is well tolerated and performance enhancing. Despite a common fear of potential risks, we did not detect any moderate or severe adverse events in almost 2000 training sessions. To further objectify improvements in physical performance, quality of life, and cognition in patients with glioblastoma, we initiated a prospective multicenter study that is currently recruiting (ClinicalTrials.gov Identifier: NCT05015543).
References
Weller M, van den Bent M, Preusser M, Le Rhun E, Tonn JC, Minniti G, Bendszus M, Balana C, Chinot O, Dirven L, French P, Hegi ME, Jakola AS, Platten M, Roth P, Rudà R, Short S, Smits M, Taphoorn MJB, Deimling A v., Westphal M, Soffietti R, Reifenberger G, Wick W (2021) EANO guidelines on the diagnosis and treatment of diffuse gliomas of adulthood. Nat Rev Clin Oncol 18(3):170–186. https://doi.org/10.1038/s41571-020-00447-z. Cited PubMed; PMID 33293629
Sterckx W, Coolbrandt A, Clement P, Borgenon S, Decruyenaere M, de Vleeschouwer S, Mees A, Dierckx de Casterlé B (2015) Living with a high-grade glioma: a qualitative study of patients’ experiences and care needs. Eur J Oncol Nurs 19(4):383–390 01.003 Cited in: PubMed; PMID 25697546
Christensen JF, Simonsen C, Hojman P (2018) Exercise Training in Cancer Control and Treatment. Compr Physiol 9(1):165–205. https://doi.org/10.1002/cphy.c180016. Cited in: PubMed; PMID 30549018
Gehring K, Kloek CJ, Aaronson NK, Janssen KW, Jones LW, Sitskoorn MM, Stuiver MM (2018) Feasibility of a home-based exercise intervention with remote guidance for patients with stable grade II and III gliomas: a pilot randomized controlled trial. Clin Rehabil 32(3):352–366. https://doi.org/10.1177/0269215517728326. Cited in: PubMed; PMID 28882061
Levin GT, Greenwood KM, Singh F, Tsoi D, Newton RU (2016) Exercise improves physical function and Mental Health of Brain Cancer Survivors: two exploratory Case Studies. Integr Cancer Ther 15(2):190–196. https://doi.org/10.1177/1534735415600068Cited in: PubMed; PMID 26276806
Eisenhut L, Sadeghi-Bahmani D, Gerber M, Saemann A, Staub L, Brand S, Cordier D (2022) Effects of two types of exercise training on psychological well-being, sleep and physical fitness in patients with high-grade glioma (WHO III and IV). J Psychiatr Res 151354–151364. https://doi.org/10.1016/j.jpsychires.2022.03.058Cited in: PubMed; PMID 35537372
Cormie P, Nowak AK, Chambers SK, Galvão DA, Newton RU (2015) The potential role of exercise in neuro-oncology. Front Oncol 585. https://doi.org/10.3389/fonc.2015.00085Cited in: PubMed; PMID 25905043
Sandler CX, Matsuyama M, Jones TL, Bashford J, Langbecker D, Hayes SC (2021) Physical activity and exercise in adults diagnosed with primary brain cancer: a systematic review. J Neurooncol 153(1):1–14. https://doi.org/10.1007/s11060-021-03745-3Cited in: PubMed; PMID 33907968
Schmitz KH, Courneya KS, Matthews C, Demark-Wahnefried W, Galvão DA, Pinto BM, Irwin ML, Wolin KY, Segal RJ, Lucia A, Schneider CM, Gruenigen VE v., Schwartz AL (2010) American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc 42(7):1409–1426. https://doi.org/10.1249/MSS.0b013e3181e0c112Cited in: PubMed; PMID 20559064
Boeckh-Behrens W-U, Buskies W, Fitness-Krafttraining (April 2016) Die besten Übungen und Methoden für Sport und Gesundheit. Rororo Sport, vol 19481, 17th edn. Rowohlt Taschenbuch Verlag, Reinbek bei Hamburg, p 478
Andersen KL (1971) Fundamentals of exercise testing. Geneva. World Health Organization; H.M.S.O, London, p 138
Wahlund H (1948) Determination of the physical Working Capacity: a physiological and clinical study with special reference to standardization of Cardio-pulmonary functional tests. Acta Med Scand 132(215):78–86
Rost R, Heck H, Hollmann W (1982) Belastungsuntersuchungen in der Praxis: Grundlagen, Technik und Interpretation ergometrischer Untersuchungsverfahren. Thieme, Stuttgart [etc.], p 164
Tanaka H, Monahan KD, Seals DR (2001) Age-predicted maximal heart rate revisited. J Am Coll Cardiol 37(1):153–156. https://doi.org/10.1016/s0735-1097(00)01054-8 Cited in: PubMed; PMID 11153730
Miyashita M, Mutoh Y, Yoshioka N, Sadamoto T (1985) PWC75%HRmax: a measure of aerobic work capacity. Sports Med 2(3):159–164. https://doi.org/10.2165/00007256-198502030-00001Cited in: PubMed; PMID 3848048
Louis DN, Perry A, Reifenberger G, Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD, Kleihues P, Ellison DW (2016) The 2016 World Health Organization classification of tumors of the Central Nervous System: a summary. Acta Neuropathol 131(6):803–820. https://doi.org/10.1007/s00401-016-1545-1Cited in: PubMed; PMID 27157931
Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJB, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352(10):987–996. https://doi.org/10.1056/NEJMoa043330. Cited in: PubMed; PMID 15758009
Herrlinger U, Tzaridis T, Mack F, Steinbach JP, Schlegel U, Sabel M, Hau P, Kortmann R-D, Krex D, Grauer O, Goldbrunner R, Schnell O, Bähr O, Uhl M, Seidel C, Tabatabai G, Kowalski T, Ringel F, Schmidt-Graf F, Suchorska B, Brehmer S, Weyerbrock A, Renovanz M, Bullinger L, Galldiks N, Vajkoczy P, Misch M, Vatter H, Stuplich M, Schäfer N, Kebir S, Weller J, Schaub C, Stummer W, Tonn J-C, Simon M, Keil VC, Nelles M, Urbach H, Coenen M, Wick W, Weller M, Fimmers R, Schmid M, Hattingen E, Pietsch T, Coch C, Glas M (2019) Lomustine-temozolomide combination therapy versus standard temozolomide therapy in patients with newly diagnosed glioblastoma with methylated MGMT promoter (CeTeG/NOA-09): a randomised, open-label, phase 3 trial. Lancet 393(10172):678–688. https://doi.org/10.1016/S0140-6736(18)31791-4Cited in: PubMed; PMID 30782343
van den Bogard F, Hamer HM, Sassen R, Reinsberger C (2020) Sport and Physical Activity in Epilepsy. Dtsch Arztebl Int 117(1–2):1–6. https://doi.org/10.3238/arztebl.2020.0001Cited in: PubMed; PMID 32008605
Hansen A, Søgaard K, Minet LR, Jarden JO (2018) A 12-week interdisciplinary rehabilitation trial in patients with gliomas - a feasibility study. Disabil Rehabil 40(12):1379–1385. https://doi.org/10.1080/09638288.2017.1295472Cited in: PubMed; PMID 28286968
Hansen A, Pedersen CB, Jarden JO, Beier D, Minet LR, Søgaard K (2020) Effectiveness of physical therapy- and occupational therapy-based Rehabilitation in People who have glioma and are undergoing active Anticancer treatment: Single-Blind, randomized controlled trial. Phys Ther 100(3):564–574. https://doi.org/10.1093/ptj/pzz180Cited in: PubMed; PMID 32043148
Spencer J, Staffileno BA (2021) Exercise intervention: a pilot study to assess the feasibility and impact on Cancer-Related fatigue and quality of life among patients with High-Grade Glioma. Clin J Oncol Nurs 25(2):194–200. https://doi.org/10.1188/21.CJON.194-200Cited in: PubMed; PMID 33739350
Ayotte SL, Harro CC (2017) Effects of an Individualized Aerobic Exercise Program in individuals with a brain tumor undergoing Inpatient Rehabilitation: a feasibility study. Rehabilitation Oncol 35(4):163–171. https://doi.org/10.1097/01.REO.0000000000000069
Capozzi LC, Boldt KR, Easaw J, Bultz B, Culos-Reed SN (2016) Evaluating a 12-week exercise program for brain cancer patients. Psychooncology 25(3):354–358. https://doi.org/10.1002/pon.3842Cited in: PubMed; PMID 25994321
Lavín-Pérez AM, Collado-Mateo D, Mayo X, Liguori G, Humphreys L, Copeland RJ, Jiménez A (2021) Effects of high-intensity training on the quality of life of cancer patients and survivors: a systematic review with meta-analysis. Sci Rep 11(1):15089. https://doi.org/10.1038/s41598-021-94476-yPMID 34301995
Troschel FM, Brandt R, Wiewrodt R, Stummer W, Wiewrodt D (2019) High-Intensity Physical Exercise in a Glioblastoma patient under Multimodal Treatment. Med Sci Sports Exerc 51(12):2429–2433. https://doi.org/10.1249/MSS.0000000000002067Cited in: PubMed; PMID 31730561
Sweegers MG, Altenburg TM, Chinapaw MJ, Kalter J, Verdonck-de Leeuw IM, Courneya KS, Newton RU, Aaronson NK, Jacobsen PB, Brug J, Buffart LM (2018) Which exercise prescriptions improve quality of life and physical function in patients with cancer during and following treatment? A systematic review and meta-analysis of randomised controlled trials. Br J Sports Med 52(8):505–513. https://doi.org/10.1136/bjsports-2017-097891
Friedenreich CM, Stone CR, Cheung WY, Hayes SC (2020) Physical activity and mortality in Cancer Survivors: a systematic review and Meta-analysis. JNCI Cancer Spectr 4(1):pkz080. https://doi.org/10.1093/jncics/pkz080Cited in: PubMed; PMID 32337494
Courneya KS, Segal RJ, Mackey JR, Gelmon K, Reid RD, Friedenreich CM, Ladha AB, Proulx C, Vallance JKH, Lane K, Yasui Y, McKenzie DC (2007) Effects of aerobic and resistance exercise in breast cancer patients receiving adjuvant chemotherapy: a multicenter randomized controlled trial. J Clin Oncol 25(28):4396–4404 08.2024 Cited in: PubMed; PMID 17785708
Courneya KS, Segal RJ, McKenzie DC, Dong H, Gelmon K, Friedenreich CM, Yasui Y, Reid RD, Crawford JJ, Mackey JR (2014) Effects of exercise during adjuvant chemotherapy on breast cancer outcomes. Med Sci Sports Exerc 46(9):1744–1751 .0000000000000297 Cited in: PubMed; PMID 24633595
Holmes MD, Chen WY, Feskanich D, Kroenke CH, Colditz GA (2005) Physical activity and survival after breast cancer diagnosis. JAMA 293(20):2479–2486. https://doi.org/10.1001/jama.293.20.2479Cited in: PubMed; PMID 15914748
Haydon AMM, Macinnis RJ, English DR, Giles GG (2006) Effect of physical activity and body size on survival after diagnosis with colorectal cancer. Gut 55(1):62–67. https://doi.org/10.1136/gut.2005.068189Cited in: PubMed; PMID 15972299
Meyerhardt JA, Giovannucci EL, Ogino S, Kirkner GJ, Chan AT, Willett W, Fuchs CS (2009) Physical activity and male colorectal cancer survival. Arch Intern Med 169(22):2102–2108. https://doi.org/10.1001/archinternmed.2009.412Cited in: PubMed; PMID 20008694
Meyerhardt JA, Giovannucci EL, Holmes MD, Chan AT, Chan JA, Colditz GA, Fuchs CS (2006) Physical activity and survival after colorectal cancer diagnosis. J Clin Oncol 24(22):3527–3534. https://doi.org/10.1200/JCO.2006.06.0855Cited in: PubMed; PMID 16822844
Hayes BD, Brady L, Pollak M, Finn SP (2016) Exercise and prostate Cancer: evidence and proposed mechanisms for Disease Modification. Cancer Epidemiol Biomarkers Prev 25(9):1281–1288. https://doi.org/10.1158/1055-9965.EPI-16-0223Cited in: PubMed; PMID 27389872
Furtner J, Weller M, Weber M, Gorlia T, Nabors B, Reardon DA, Tonn JC, Stupp R, Preusser M (2022) Temporal muscle thickness as a prognostic marker in patients with newly diagnosed Glioblastoma: translational imaging analysis of the CENTRIC EORTC 26071 – 22072 and CORE trials. Clin Cancer Res 28(1):129–136. https://doi.org/10.1158/1078-0432.CCR-21-1987Cited in: PubMed; PMID 34667022
Steindl A, Leitner J, Schwarz M, Nenning K-H, Asenbaum U, Mayer S, Woitek R, Weber M, Schöpf V, Berghoff AS, Berger T, Widhalm G, Prayer D, Preusser M, Furtner J (2020) Sarcopenia in neurological patients: standard values for temporal muscle thickness and muscle strength evaluation. J Clin Med 9(5). https://doi.org/10.3390/jcm9051272Cited in: PubMed; PMID 32354003
Costa AK, Marqueze LFB, Gattiboni BB, Pedroso GS, Vasconcellos FF, Cunha EBB, Justa HC, Baldissera AB, Nagashima S, de Noronha L, Radak Z, Fernandes LC, Pinho RA (2022) Physical training protects against brain toxicity in mice exposed to an experimental model of Glioblastoma. Neurochem Res 47(11):3344–3354. https://doi.org/10.1007/s11064-022-03685-y Cited in: PubMed; PMID 35904698
Lemke D, Pledl H-W, Zorn M, Jugold M, Green E, Blaes J, Löw S, Hertenstein A, Ott M, Sahm F, Steffen A-C, Weiler M, Winkler F, Platten M, Dong Z, Wick W (2016) Slowing down glioblastoma progression in mice by running or the anti-malarial drug dihydroartemisinin? Induction of oxidative stress in murine glioblastoma therapy. Oncotarget 7(35):56713–56725. https://doi.org/10.18632/oncotarget.10723Cited in: PubMed; PMID 27447560
Tantillo E, Colistra A, Baroncelli L, Costa M, Caleo M, Vannini E (2020) Voluntary Physical Exercise reduces Motor Dysfunction and hampers Tumor Cell Proliferation in a mouse model of Glioma. Int J Environ Res Public Health 17(16). https://doi.org/10.3390/ijerph17165667Cited in: PubMed; PMID 32764487
Finger JD, Krug S, Gößwald A, Härtel S, Bös K (2013) Kardiorespiratorische Fitness bei Erwachsenen in Deutschland: Ergebnisse der Studie zur Gesundheit Erwachsener in Deutschland (DEGS1) [Cardiorespiratory fitness among adults in Germany: results of the German Health Interview and Examination Survey for Adults (DEGS1)]. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. ;56(5–6):772–8. ger. https://doi.org/10.1007/s00103-013-1672-y. Cited in: PubMed; PMID 23703497
Acknowledgements
We are very grateful for the continuous support of the Münster University Hospital Central Nervous System Association (Förderverein Zentrales Nervensystem e.V.) represented by Alice Kraul and Hansdetlef Wassmann M.D., Ph.D.
Funding
This research was funded by the Münster University Hospital Central Nervous System Association (Förderverein Zentrales Nervensystem e.V.). The funding allowed patients to participate in the intervention free of charge. W.S., K.V., and R.W. are endowed by the Medical Faculty of the Westfalian Wilhelm-University, Münster.
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
J.J., M.M., R.W., and D.W. contributed equally to this work and should be considered co-first authors (J.J. and M.M.) and co-last authors (R.W. and D.W.).Parts of the analysis presented are taken from the doctoral thesis of U.A.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of Interest
The authors do not report potential conflicts of interest.
Financial disclosure
There are no financial interests to disclose.
Authorship statement
J.J., M.M., R.W., and D.W. contributed equally to this work and should be considered co-first authors (J.J. and M.M.) and co-last authors (R.W. and D.W.). Parts of the analysis presented are taken from the doctoral thesis of U.A.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jost, J., Müther, M., Brandt, R. et al. Conceptual development of an intensive exercise program for glioma patients (ActiNO): summary of clinical experience. J Neurooncol 163, 367–376 (2023). https://doi.org/10.1007/s11060-023-04354-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-023-04354-y