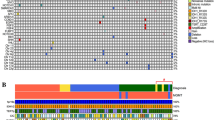
Abstract
Purpose
Astrocytomas are a type of malignant brain tumor with an unfavorable clinical course. The impact of AGT and MGMT somatic variants in the prognosis of astrocytoma is unknown, and it is controversial for TP53. Moreover, there is a lack of knowledge regarding the molecular characteristics of astrocytomas in Mexican patients.
Methods
We studied 48 Mexican patients, men and women, with astrocytoma (discovery cohort). We performed DNA deep sequencing in tumor samples, targeting AGT, MGMT and TP53, and we studied MGMT gene promoter methylation status. Then we compared our findings to a cohort which included data from patients with astrocytoma from The Cancer Genome Atlas (validation cohort).
Results
In the discovery cohort, we found a higher number of somatic variants in AGT and MGMT than in the validation cohort (10.4% vs < 1%, p < 0.001), and, in both cohorts, we observed only women carried variants AGT variants. We also found that the presence of either MGMT variant or promoter methylation was associated to better survival and response to chemotherapy, and, in conjunction with TP53 variants, to progression-free survival.
Conclusions
The occurrence of AGT variants only in women expands our knowledge about the molecular differences in astrocytoma between men and women. The increased prevalence of AGT and MGMT variants in the discovery cohort also points towards possible distinctions in the molecular landscape of astrocytoma among populations. Our findings warrant further study.

Similar content being viewed by others
Data availability
DNA sequences from the discovery cohort were deposited at NCBI’s Sequence Read Archive (Bioproject PRJNA862290). TCGA data was obtained from https://xenabrowser.net/datapages/, TCGA Glioblastoma and TCGA Lower Grade Glioma.
References
Ostrom QT, Cioffi G, Waite K, Kruchko C, Barnholtz-Sloan JS (2021) CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2014–2018. Neuro Oncol 23(12):31–3105. https://doi.org/10.1093/neuonc/noab200
Neth B, Carabenciov I, Ruff M, Johnson D (2021) Temporal trends in glioblastoma survival progress then plateau. Neurologist 27(3):119–124. https://doi.org/10.1097/NRL.0000000000000393
Dong X, Noorbakhsh A, Hirshman BR et al (2014) Survival trends of grade I, II, and III astrocytoma patients and associated clinical practice patterns between 1999 and 2010: a SEER-based analysis. Neuro-Oncol Pract 3(1):29–38. https://doi.org/10.1093/nop/npv016
Hegi ME, Diserens A-C, Gorlia T et al (2005) MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med 352(10):997–1003. https://doi.org/10.1056/NEJMoa043331
Weller M, van den Bent M, Preusser M et al (2021) EANO guidelines on the diagnosis and treatment of diffuse gliomas of adulthood. Nat Rev Clin Oncol 18(3):170–186. https://doi.org/10.1038/s41571-020-00447-z
Yu W, Zhang L, Wei Q, Shao A (2020) O6-methylguanine-DNA methyltransferase (MGMT): challenges and new opportunities in glioma chemotherapy. Front Oncol 9(January):1–11. https://doi.org/10.3389/fonc.2019.01547
Rapkins RW, Wang F, Nguyen HN et al (2015) The MGMT promoter SNP rs16906252 is a risk factor for MGMT methylation in glioblastoma and is predictive of response to temozolomide. Neuro Oncol 17(12):1589–1598. https://doi.org/10.1093/neuonc/nov064
Perdomo-Pantoja A, Mejía-Pérez SI, Gómez-Flores-Ramos L et al (2018) Renin angiotensin system and its role in biomarkers and treatment in gliomas. J Neurooncol 138(1):1–15. https://doi.org/10.1007/s11060-018-2789-5
Urup T, Michaelsen SR, Olsen LR et al (2016) Angiotensinogen and HLA class II predict bevacizumab response in recurrent glioblastoma patients. Mol Oncol 10(8):1160–1168. https://doi.org/10.1016/j.molonc.2016.05.005
Urup T, Gillberg L, Kaastrup K et al (2020) Angiotensinogen promoter methylation predicts bevacizumab treatment response of patients with recurrent glioblastoma. Mol Oncol. https://doi.org/10.1002/1878-0261.12660
Perdomo-Pantoja A, Mejía-Pérez SI, Reynoso-Noverón N et al (2018) Angiotensinogen rs5050 germline genetic variant as potential biomarker of poor prognosis in astrocytoma. PLoS ONE 13(11):e0206590. https://doi.org/10.1371/journal.pone.0206590
Brennan CW, Verhaak RGW, McKenna A et al (2013) The somatic genomic landscape of glioblastoma. Cell 155(2):462–477. https://doi.org/10.1016/J.CELL.2013.09.034
The Cancer Genome Atlas Research Network (2008) Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature 455(7216):1061–1068. https://doi.org/10.1038/nature07385
Bäcklund LM, Nilsson BR, Liu L, Ichimura K, Collins VP (2005) Mutations in Rb1 pathway-related genes are associated with poor prognosis in anaplastic astrocytomas. Br J Cancer 93(1):124–130. https://doi.org/10.1038/sj.bjc.6602661
Ständer M, Peraud A, Leroch B, Kreth FW (2004) Prognostic impact of TP53 mutation status for adult patients with supratentorial World Health Organization Grade II astrocytoma or oligoastrocytoma. Cancer 101(5):1028–1035. https://doi.org/10.1002/cncr.20432
Gillet E, Alentorn A, Doukouré B et al (2014) TP53 and p53 statuses and their clinical impact in diffuse low grade gliomas. J Neurooncol 118(1):131–139. https://doi.org/10.1007/s11060-014-1407-4
Wang X, Chen JX, Liu JP, You C, Liu YH, Mao Q (2014) Gain of function of mutant TP53 in glioblastoma: prognosis and response to temozolomide. Ann Surg Oncol 21(4):1337–1344. https://doi.org/10.1245/s10434-013-3380-0
Stancheva G, Goranova T, Laleva M et al (2014) IDH1/IDH2 but not TP53 mutations predict prognosis in Bulgarian glioblastoma patients. Biomed Res Int 2014:1–9. https://doi.org/10.1155/2014/654727
Wegman-Ostrosky T, Reynoso-Noverón N, Mejía-Pérez SI et al (2016) Clinical prognostic factors in adults with astrocytoma: Historic cohort. Clin Neurol Neurosurg 146:116–122. https://doi.org/10.1016/j.clineuro.2016.05.002
Beltrán JQ, Soto-Abraham JE, Vidaurreta-Serrano J, Chavez-Macias LG, Gómez-Apo E, Ogando-Rivas E (2018) Astrocytic tumors in Mexico: an overview of characteristics and prognosis in an open reference center for low-income population. J Neurosci Rural Pract 9(4):516–521
Lopez González MA, Sotelo J (2000) Brain tumors in Mexico: characteristics and prognosis of glioblastoma. Surg Neurol 53(2):157–162
McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM (2005) REporting recommendations for tumour MARKer prognostic studies (REMARK). Br J Cancer 93(4):387–391. https://doi.org/10.1038/sj.bjc.6602678
Carlos-Escalante JA, Bian X, Perdomo-Pantoja A et al (2020) Landscape of germline genetic variants in AGT, MGMT, and TP53 in Mexican adult patients with astrocytoma. Cell Mol Neurobiol. https://doi.org/10.1007/s10571-020-00901-7
Deshpande A, Lang W, McDowell T et al (2018) Strategies for identification of somatic variants using the Ion Torrent deep targeted sequencing platform. BMC Bioinform 19(1):1–10. https://doi.org/10.1186/s12859-017-1991-3
Liu X, Li C, Mou C, Dong Y, Tu Y (2020) dbNSFP v4: a comprehensive database of transcript-specific functional predictions and annotations for human nonsynonymous and splice-site SNVs. Genome Med 12(1):1–8. https://doi.org/10.1186/s13073-020-00803-9
Kato S, Han S-Y, Liu W et al (2003) Understanding the function-structure and function-mutation relationships of p53 tumor suppressor protein by high-resolution missense mutation analysis. Proc Natl Acad Sci USA 100(14):8424–8429. https://doi.org/10.1073/pnas.1431692100
Sangrador-Deitos MV, Villanueva-Castro E, Marian-Magaña R et al (2022) Carboplatin plus vincristine as an alternative chemotherapeutic scheme in patients with glioblastoma. Cureus. https://doi.org/10.7759/cureus.24467
Zhang H, Liao J, Zhang X et al (2019) Sex difference of mutation clonality in diffuse glioma evolution. Neuro Oncol 21(2):201–213
Greenland KJ, Sernia C (2004) Oestrogenic regulation of brain angiotensinogen. J Neuroendocrinol 16(6):508–515. https://doi.org/10.1111/j.1365-2826.2004.01194.x
Balam-Ortiz E, Esquivel-Villarreal A, Alfaro-Ruiz L et al (2011) Variants and haplotypes in angiotensinogen gene are associated with plasmatic angiotensinogen level in Mexican population. Am J Med Sci 342(3):205–211. https://doi.org/10.1097/MAJ.0b013e3182121020
Ursu R, Thomas L, Psimaras D et al (2019) Angiotensin II receptor blockers, steroids and radiotherapy in glioblastoma: a randomised multicentre trial (ASTER trial) An ANOCEF study. Eur J Cancer 109:129–136. https://doi.org/10.1016/j.ejca.2018.12.025
Sheng Z, Kang M, Wang H (2018) The potential role of MGMT rs12917 polymorphism in cancer risk: an updated pooling analysis with 21010 cases and 34018 controls. Biosci Rep. https://doi.org/10.1042/BSR20180942
Hill CE, Wickliffe JK, Guerin AT et al (2007) The L84F polymorphism in the O6-Methylguanine-DNA-Methyltransferase (MGMT) gene is associated with increased hypoxanthine phosphoribosyltransferase (HPRT) mutant frequency in lymphocytes of tobacco smokers. Pharmacogenet Genom 17(9):743–753. https://doi.org/10.1097/FPC.0b013e3281111eb1
Molina E, Pérez-Morales R, Rubio J et al (2013) The GSTM1null (deletion) and MGMT84 rs12917 (Phe/Phe) haplotype are associated with bulky DNA adduct levels in human leukocytes. Mutat Res Genet Toxicol Environ Mutagen 758(1–2):62–68. https://doi.org/10.1016/j.mrgentox.2013.09.007
Adegboyega G, Ozair A, Kanmounye US, Bandyopadhyay S, Vaqas B (2021) Letter: is the stupp protocol an expensive and unsustainable standard of care for glioblastoma in low- and middle-income country settings? A call to action! Neurosurgery 89(4):E249–E251. https://doi.org/10.1093/neuros/nyab273
Okamoto Y, Di Patre P-L, Burkhard C et al (2004) Population-based study on incidence, survival rates, and genetic alterations of low-grade diffuse astrocytomas and oligodendrogliomas. Acta Neuropathol 108(1):49–56. https://doi.org/10.1007/s00401-004-0861-z
Arita H, Narita Y, Fukushima S et al (2013) Upregulating mutations in the TERT promoter commonly occur in adult malignant gliomas and are strongly associated with total 1p19q loss. Acta Neuropathol 126(2):267–276. https://doi.org/10.1007/s00401-013-1141-6
Cardona AF, Rojas L, Wills B et al (2016) Genotyping low-grade gliomas among Hispanics. Neuro-Oncol Pract 3(3):164–172. https://doi.org/10.1093/nop/npv061
Noor H, Briggs NE, McDonald KL, Holst J, Vittorio O (2021) Tp53 mutation is a prognostic factor in lower grade glioma and may influence chemotherapy efficacy. Cancers (Basel) 13(21):5362. https://doi.org/10.3390/cancers13215362
Wang K, Wang YY, Ma J et al (2014) Prognostic value of MGMT promoter methylation and TP53 mutation in glioblastomas depends on IDH1 mutation. Asian Pacific J Cancer Prev 15(24):10893–10898. https://doi.org/10.7314/APJCP.2014.15.24.10893
Yang K, Jung SW, Shin H et al (2019) Cancer genetic markers according to radiotherapeutic response in patients with primary glioblastoma–Radiogenomic approach for precision medicine. Radiother Oncol 131:66–74. https://doi.org/10.1016/j.radonc.2018.11.025
Weller M, Felsberg J, Hartmann C et al (2009) Molecular predictors of progression-free and overall survival in patients with newly diagnosed glioblastoma: a prospective translational study of the German Glioma Network. J Clin Oncol 27(34):5743–5750. https://doi.org/10.1200/JCO.2009.23.0805
Spratt DE, Chan T, Waldron L et al (2016) Racial/ethnic disparities in genomic sequencing. JAMA Oncol 2(8):E1–E5. https://doi.org/10.1001/jamaoncol.2016.1854
Yang W, Warrington NM, Taylor SJ et al (2019) Sex differences in GBM revealed by analysis of patient imaging, transcriptome, and survival data. Sci Transl Med. https://doi.org/10.1126/scitranslmed.aao5253
Sun T, Warrington NM, Luo J et al (2014) Sexually dimorphic RB inactivation underlies mesenchymal glioblastoma prevalence in males. J Clin Invest 124(9):4123–4133. https://doi.org/10.1172/JCI71048
Acknowledgements
We sincerely thank Laura Márquez, Patricia de la Torre and Patricia Rosas for their contributions in the project. Many thanks to the PECEM and the Universidad Nacional Autónoma de México (UNAM) for their support of this project.
Funding
This research was funded by the Consejo Nacional de Ciencia y Tecnología (CONACyT), Mexico: Salud 2013-01-202720 to TWO. Scolarship number 969754 to JACE).
Author information
Authors and Affiliations
Contributions
Conceptualization: TWO, SVM, TC, POW, SIMP, and BCD. Data Curation: JACE, SI MP, TWO. Formal Analysis: JACE. Funding Acquisition: TWO, AMB, and LAH. Investigation: ESR, TWO, LGC, SIMP, JACE, KTA, MMC, RMAG, AMSM, APC, JASL, CHPC, TESC, MRC, and CEDV. Methodology: JACE, SIMP and TWO. Resources: TWO, CEDV, RGB, CAC, FVP, POW. Supervision: TWO, CAC and FVP. Validation: JACE, NRN, MRC, and CEDV. Visualization: JSCE. Writing – Original Draft: JA CE, AGA, and TWO. Writing – Review & Editing: All Authors.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Consent to participate
Written informed consent was obtained from all individuals participating in the study.
Consent to publish
We confirm that all participants provided informed consent for publication of anonymized data in Online Resource 4.
Ethical approval
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Board Review of the Instituto National de Neurología y Neurocirugía, registry number 67/12.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Carlos-Escalante, J.A., Mejía-Pérez, S.I., Soto-Reyes, E. et al. Deep DNA sequencing of MGMT, TP53 and AGT in Mexican astrocytoma patients identifies an excess of genetic variants in women and a predictive biomarker. J Neurooncol 161, 165–174 (2023). https://doi.org/10.1007/s11060-022-04214-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-022-04214-1