Abstract
Purpose
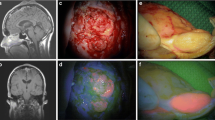
5-aminolevulinic acid (5-ALA) has demonstrated its utility as an intraoperative imaging adjunct during fluorescence guided resection of malignant gliomas. However, literature regarding 5-ALA-guided resection for brain metastases is limited. We conducted a systematic review to evaluate the efficacy of 5-ALA fluorescence for resection of metastatic brain tumors.
Methods
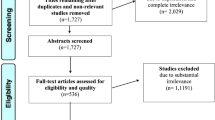
PubMed was queried for studies involving 5-ALA and brain metastases, and results were screened following PRISMA guidelines. Articles related to 5-ALA and brain metastasis were further assessed based on inclusion and exclusion criteria and results were analyzed for 5-ALA fluorescence rates stratified by tumor primary sites and histological subtypes.
Results
Of 421 identified search results, 10 studies were included and a total of 631 patients analyzed. Of these studies, 60% were retrospective in design. The reported rates of 5-ALA fluorescence in included brain metastases ranged from 27.6 to 86.9%, with variability across and within tumor types. No studies concluded improved operative outcomes or survival outcomes related to 5-ALA use.
Conclusions
Current studies regarding 5-ALA fluorescence in brain metastases are limited and do not confirm efficacy for improving extent of resection or post-operative survival. Fluorescence is variable across and within tumor types. Further studies are necessary to evaluate whether specific tumors may benefit from 5-ALA FGS or if changes in delivery protocols or fluorescence quantification may affect intraoperative utility.


Similar content being viewed by others
References
Patchell RA et al (1998) Postoperative radiotherapy in the treatment of single metastases to the brain: a randomized trial. JAMA 280(17):1485–1489
Nayak L, Lee EQ, Wen PY (2012) Epidemiology of brain metastases. Curr Oncol Rep 14(1):48–54
Hatiboglu MA, Akdur K, Sawaya R (2020) Neurosurgical management of patients with brain metastasis. Neurosurg Rev 43(2):483–495
Lowery FJ, Yu D (2017) Brain metastasis: unique challenges and open opportunities. Biochim Biophys Acta (BBA) 1867(1):49–57
Stelzer KJ (2013) Epidemiology and prognosis of brain metastases. Surg Neurol Int 4:S192
Kotecha R et al (2018) Recent advances in managing brain metastasis. F1000Res 7:F1000
Vogelbaum MA et al (2021) Treatment for brain metastases: ASCO-SNO-ASTRO guideline. J Clin Oncol 40(5):492–516
Winther RR et al (2022) Surgery for brain metastases-impact of the extent of resection. Acta Neurochir 164(10):2773–2780
Siam L et al (2015) The metastatic infiltration at the metastasis/brain parenchyma-interface is very heterogeneous and has a significant impact on survival in a prospective study. Oncotarget 6(30):29254–29267
Berghoff AS et al (2013) Invasion patterns in brain metastases of solid cancers. Neuro Oncol 15(12):1664–1672
Weller M et al (2021) EANO guidelines on the diagnosis and treatment of diffuse gliomas of adulthood. Nat Rev Clin Oncol 18(3):170–186
Zhao S et al (2013) Intraoperative fluorescence-guided resection of high-grade malignant gliomas using 5-aminolevulinic acid-induced porphyrins: a systematic review and meta-analysis of prospective studies. PLoS ONE 8(5):e63682
Dadario NB et al (2021) 5-aminolevulinic acid-shedding light on where to focus. World Neurosurg 150:9–16
Stummer W et al (2006) Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol 7(5):392–401
Duffner F et al (2005) Specific intensity imaging for glioblastoma and neural cell cultures with 5-aminolevulinic acid-derived protoporphyrin IX. J Neurooncol 71(2):107–111
Stummer W et al (1998) In vitro and in vivo porphyrin accumulation by C6 glioma cells after exposure to 5-aminolevulinic acid. J Photochem Photobiol B 45(2–3):160–169
Gandhi S et al (2019) Survival outcomes among patients with high-grade glioma treated with 5-aminolevulinic acid-guided surgery: a systematic review and meta-analysis. Front Oncol 9:620
Baig Mirza A et al (2021) 5-Aminolevulinic acid-guided resection improves the overall survival of patients with glioblastoma-a comparative cohort study of 343 patients. Neurooncol Adv. 3(1):vdab047
Mansouri A et al (2016) The role of 5-aminolevulinic acid in enhancing surgery for high-grade glioma, its current boundaries, and future perspectives: a systematic review. Cancer 122(16):2469–2478
Teng L et al (2011) Silencing of ferrochelatase enhances 5-aminolevulinic acid-based fluorescence and photodynamic therapy efficacy. Br J Cancer 104:798–807
Solomou G et al (2022) Utility of 5-ALA for resection of CNS tumours other than high-grade gliomas: a protocol for a systematic review. BMJ Open 12(7):e056059
Ferraro N et al (2016) The role of 5-aminolevulinic acid in brain tumor surgery: a systematic review. Neurosurg Rev 39(4):545–555
Ahrens LC et al (2022) Effect of 5-aminolevulinic acid and sodium fluorescein on the extent of resection in high-grade gliomas and brain metastasis. Cancers (Basel) 14(3):617
Guyotat J et al (2016) 5-aminolevulinic acid-protoporphyrin IX fluorescence-guided surgery of high-grade gliomas: a systematic review. Adv Tech Stand Neurosurg 43:61–90
Marhold F et al (2022) Does pigmentation, hemosiderin and blood effect visible 5-ALA fluorescence in cerebral melanoma metastasis? Photodiagn Photodyn Ther 39:102864
Zimm S, Wampler GL, Stablein D, Hazra T, Young HF (1981) Intracerebral metastases in solid-tumor patients: natural history and results of treatment. Cancer 48(2):384–394
Yoo H et al (2009) Reduced local recurrence of a single brain metastasis through microscopic total resection: clinical article. J Neurosurg JNS 110(4):730–736
Neves S et al (2001) Pseudogliomatous growth pattern of anaplastic small cell carcinomas metastatic to the brain. Clin Neuropathol 20(1):38–42
Baumert BG et al (2006) A pathology-based substrate for target definition in radiosurgery of brain metastases. Int J Radiat Oncol Biol Phys 66(1):187–194
Hussein A et al (2020) Survival after resection of brain metastases with white light microscopy versus fluorescence-guidance: a matched cohort analysis of the Metastasys study data. Oncotarget 11(32):3026–3034
Kamp MA et al (2012) 5-Aminolevulinic acid (5-ALA)-induced fluorescence in intracerebral metastases: a retrospective study. Acta Neurochir 154(2):223–228
Schatlo B et al (2019) 5-aminolevulinic acid fluorescence indicates perilesional brain infiltration in brain metastases. World Neurosurg X 5:100069
Masubuchi T et al (2013) Experimental study to understand nonspecific protoporphyrin IX fluorescence in brain tissues near tumors after 5-aminolevulinic acid administration. Photomed Laser Surg 31(9):428–433
Miyatake S et al (2007) Fluorescence of non-neoplastic, magnetic resonance imaging-enhancing tissue by 5-aminolevulinic acid: case report. Neurosurgery 61(5):E1101-3 (discussion E1103-4)
Kamp MA et al (2018) Various shades of red-a systematic analysis of qualitative estimation of ALA-derived fluorescence in neurosurgery. Neurosurg Rev 41(1):3–18
Marhold F et al (2020) Detailed analysis of 5-aminolevulinic acid induced fluorescence in different brain metastases at two specialized neurosurgical centers: experience in 157 cases. J Neurosurg 133(4):1032–1043
Ji SY, Kim JW, Park CK (2019) Experience profiling of fluorescence-guided surgery II: non-glioma pathologies. Brain Tumor Res Treat 7(2):98–104
Stummer W et al (2019) Intraoperative fluorescence diagnosis in the brain: a systematic review and suggestions for future standards on reporting diagnostic accuracy and clinical utility. Acta Neurochir (Wien) 161(10):2083–2098
Knipps J et al (2019) Quantification of PpIX-fluorescence of cerebral metastases: a pilot study. Clin Exp Metas 36(5):467–475
Widhalm G et al (2019) The value of visible 5-ALA fluorescence and quantitative protoporphyrin IX analysis for improved surgery of suspected low-grade gliomas. J Neurosurg 133:1–10
Omoto K et al (2019) Expression of peptide transporter 1 has a positive correlation in protoporphyrin IX accumulation induced by 5-aminolevulinic acid with photodynamic detection of non-small cell lung cancer and metastatic brain tumor specimens originating from non-small cell lung cancer. Photodiagn Photodyn Ther 25:309–316
Hagiya Y et al (2011) Pivotal roles of peptide transporter PEPT1 and ATP-binding cassette (ABC) transporter ABCG2 in 5-aminolevulinic acid (ALA)-based photocytotoxicity of gastric cancer cells in vitro. Photodiagn Photodyn Ther 9(3):204–214
Hagiya Y et al (2013) Expression levels of PEPT1 and ABCG2 play key roles in 5-aminolevulinic acid (ALA)-induced tumor-specific protoporphyrin IX (PpIX) accumulation in bladder cancer. Photodiagn Photodyn Ther 10(3):288–295
Utsuki S et al (2007) Fluorescence-guided resection of metastatic brain tumors using a 5-aminolevulinic acid-induced protoporphyrin IX: pathological study. Brain Tumor Pathol 24:53
Drug Approval Package: Gleolan (aminolevulinic acid hydrochloride)
Suero Molina E et al (2022) Double dose of 5-aminolevulinic acid and its effect on protoporphyrin IX accumulation in low-grade glioma. J Neurosurg 137(4):943–952
Kamp MA et al (2012) Is it a glioblastoma? In dubio pro 5-ALA! Acta Neurochir 154(7):1269–1273
Acknowledgements
N/A.
Funding
The authors declare that no financial support was received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Data collection, analysis, and the first draft were prepared by HS, SL, and HK. All authors commented on previous versions of the draft. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors have no competing financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shah, H.A., Leskinen, S., Khilji, H. et al. Utility of 5-ALA for fluorescence-guided resection of brain metastases: a systematic review. J Neurooncol 160, 669–675 (2022). https://doi.org/10.1007/s11060-022-04188-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-022-04188-0