Abstract
Introduction
Ultrasound (US) is a versatile technology, able to provide a real-time and multiparametric intraoperative imaging, and a promising way to treat neuro-oncological patients outside the operating room. Anyhow, its potential is limited both in imaging and therapeutic purposes by the existence of the bone shielding. To enhance the spectrum of uses, our group has designed a dedicated US-translucent cranial prosthesis. Herein, we provide the proof of concept of a long-term US-based follow-up and a potential bedside therapeutic exploitation of US.
Methods
The prosthesis was first implanted in a cadaveric specimen to record any issue related to the cranioplasty procedure. Hence, the device was implanted in a patient undergoing surgery for a multi-recurrent anaplastic oligodendroglioma. US multiparametric scans through the device were acquired at 3, 6, 9, and 30 months after the procedure.
Results
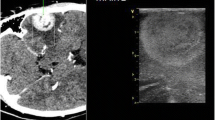
The prosthesis could be modeled and implanted through ordinary instruments, with no concerns over safety and feasibility. Trans-prosthesis multiparametric US imaging was feasible, with image quality comparable to intraoperative US. Long-term follow-up in an outpatient setting was possible with no adverse events. Trans-prosthesis mechanical interaction with microbubbles was also feasible during follow-up.
Conclusions
This report provides the first proof of concept for a potential breakthrough in the management of neuro-oncological patients. Indeed, through the implantation of an artificial acoustic window, the road is set to employ US both for a more dynamic long-term follow-up, and for US-guided therapeutic applications.





Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article.
References
Erdoan N, Tucer B, Mavl E et al (2005) Ultrasound guidance in intracranial tumor resection: Correlation with postoperative magnetic resonance findings. Acta radiol 46:743–749. https://doi.org/10.1080/02841850500223208
Serra C, Stauffer A, Actor B et al (2012) Intraoperative high frequency ultrasound in intracerebral high-grade tumors. Ultraschall der Medizin 33:E306–E312. https://doi.org/10.1055/s-0032-1325369
Coburger J, Scheuerle A, Kapapa T et al (2015) Sensitivity and specificity of linear array intraoperative ultrasound in glioblastoma surgery: a comparative study with high field intraoperative MRI and conventional sector array ultrasound. Neurosurg Rev 38:499–509. https://doi.org/10.1007/s10143-015-0627-1
Coburger J, König RW, Scheuerle A et al (2014) Navigated high frequency ultrasound: Description of technique and clinical comparison with conventional intracranial ultrasound. World Neurosurg 82:366–375. https://doi.org/10.1016/j.wneu.2014.05.025
Mahboob S, McPhillips R, Qiu Z et al (2016) Intraoperative ultrasound-guided resection of gliomas: a meta-analysis and review of the literature. World Neurosurg 92:255–263. https://doi.org/10.1016/j.wneu.2016.05.007
Sæther CA, Torsteinsen M, Torp SH et al (2012) Did survival improve after the implementation of intraoperative neuronavigation and 3d ultrasound in glioblastoma surgery? A retrospective analysis of 192 primary operations. J Neurol Surgery A 73:73–78. https://doi.org/10.1055/s-0031-1297247
Prada F, Solbiati L, Martegani A, DiMeco F (2016) Intraoperative ultrasound (IOUS) in neurosurgery: from standard B-mode to elastosonography. Springer International Publishing, Cham
Del Bene M, Perin A, Casali C et al (2018) Advanced ultrasound imaging in glioma surgery: beyond gray-scale B-mode. Front Oncol 8:576. https://doi.org/10.3389/fonc.2018.00576
Prada F, Perin A, Martegani A et al (2014) Intraoperative contrast-enhanced ultrasound for brain tumor surgery. Neurosurgery 74:542–552. https://doi.org/10.1227/NEU.0000000000000301 (Discussion 552)
Chauvet D, Imbault M, Capelle L et al (2016) In vivo measurement of brain tumor elasticity using intraoperative shear wave elastography. Ultraschall der Medizin 37:584–590. https://doi.org/10.1055/s-0034-1399152
Prada F, Del Bene M, Moiraghi A et al (2015) From grey scale B-mode to elastosonography: multimodal ultrasound imaging in meningioma surgery-pictorial essay and literature review. Biomed Res Int 2015:925729. https://doi.org/10.1155/2015/925729
Prada F, Del Bene M, Rampini A et al (2019) Intraoperative strain elastosonography in brain tumor surgery. Oper Neurosurg (Hagerstown) 17:227–236. https://doi.org/10.1093/ons/opy323
Cepeda S, Barrena C, Arrese I et al (2020) Intraoperative ultrasonographic elastography: A semi-quantitative analysis of brain tumor elasticity patterns and peritumoral region. World Nurosurg. https://doi.org/10.1016/j.wneu.2019.11.133
Cepeda S, García-García S, Arrese I et al (2021) Comparison of intraoperative ultrasound B-mode and strain elastography for the differentiation of glioblastomas from solitary brain metastases. An automated deep learning approach for image analysis. Front Oncol 10:1. https://doi.org/10.3389/fonc.2020.590756
Cepeda S, García-García S, Velasco-Casares M et al (2021) Is there a relationship between the elasticity of brain tumors, changes in diffusion tensor imaging, and histological findings? A pilot study using intraoperative ultrasound elastography. Brain Sci 11:1–13. https://doi.org/10.3390/brainsci11020271
Chan HW, Uff C, Chakraborty A et al (2021) Clinical application of shear wave elastography for assisting brain tumor resection. Front Oncol 11:1. https://doi.org/10.3389/fonc.2021.619286
Krishna V, Sammartino F, Rezai A (2018) A review of the current therapies, challenges, and future directions of transcranial focused ultrasound technology advances in diagnosis and treatment. JAMA Neurol 75:246–254. https://doi.org/10.1001/jamaneurol.2017.3129
Burgess A, Shah K, Hough O, Hynynen K (2015) Focused ultrasound-mediated drug delivery through the blood-brain barrier. Expert Rev Neurother 15:477–491. https://doi.org/10.1586/14737175.2015.1028369
Zhu L, Nazeri A, Pacia CP et al (2020) Focused ultrasound for safe and effective release of brain tumor biomarkers into the peripheral circulation. PLoS ONE 15:e0234182. https://doi.org/10.1371/journal.pone.0234182
Carpentier A, Canney M, Vignot A et al (2016) Clinical trial of blood-brain barrier disruption by pulsed ultrasound. Sci Transl Med 8:343re2. https://doi.org/10.1126/scitranslmed.aaf6086
Bunevicius A, McDannold NJ, Golby AJ (2020) Focused ultrasound strategies for brain tumor therapy. Oper Neurosurg 19:9–18. https://doi.org/10.1093/ons/opz374
Prada F, Kalani MYS, Yagmurlu K et al (2019) Applications of focused ultrasound in cerebrovascular diseases and brain tumors. Neurother J Am Soc Exp Neurother 16:67–87. https://doi.org/10.1007/s13311-018-00683-3
Le Rhun E, Preusser M, Roth P et al (2019) Molecular targeted therapy of glioblastoma. Cancer Treat Rev 80:101896. https://doi.org/10.1016/j.ctrv.2019.101896
Mainprize T, Lipsman N, Huang Y et al (2019) Blood-brain barrier opening in primary brain tumors with non-invasive MR-guided focused ultrasound: a clinical safety and feasibility study. Sci Rep 9:321. https://doi.org/10.1038/s41598-018-36340-0
Phenix CP, Togtema M, Pichardo S et al (2014) High intensity focused ultrasound technology, its scope and applications in therapy and drug delivery. J Pharm Pharm Sci 17:136–153. https://doi.org/10.18433/j3zp5f
Ammi AY, Douglas Mast T, Huang I-H et al (2008) Characterization of ultrasound propagation through ex-vivo human temporal bone HHS public access author manuscript. Ultrasound Med Biol 34:1578–1589. https://doi.org/10.1016/j.ultrasmedbio.2008.02.012
Prada F, Franzini A, Moosa S et al (2020) In vitro and in vivo characterization of a cranial window prosthesis for diagnostic and therapeutic cerebral ultrasound. J Neurosurg. https://doi.org/10.3171/2019.10.JNS191674
Franzini A, Moosa S, Servello D et al (2019) Ablative brain surgery: an overview. Int J Hyperthermia 36:64–80. https://doi.org/10.1080/02656736.2019.1616833
Chen K-T, Lin Y-J, Chai W-Y et al (2020) Neuronavigation-guided focused ultrasound (NaviFUS) for transcranial blood-brain barrier opening in recurrent glioblastoma patients: clinical trial protocol. Ann Transl Med 8:673–673. https://doi.org/10.21037/ATM-20-344
Spena G, Guerrini F, Grimod G et al (2019) Polymethyl methacrylate cranioplasty is an effective ultrasound window to explore intracranial structures: preliminary experience and future perspectives. World Neurosurg 127:e1013–e1019. https://doi.org/10.1016/j.wneu.2019.04.026
Belzberg M, Ben SN, Yuhanna E et al (2019) Sonolucent cranial implants: cadaveric study and clinical findings supporting diagnostic and therapeutic transcranioplasty ultrasound. J Craniofac Surg 30:1456–1461. https://doi.org/10.1097/SCS.0000000000005454
Belzberg M, Ben SN, Lu A et al (2019) Transcranioplasty ultrasound through a sonolucent cranial implant made of polymethyl methacrylate: phantom study comparing ultrasound, computed tomography, and magnetic resonance imaging. J Craniofac Surg 30:E626–E629. https://doi.org/10.1097/SCS.0000000000005651
Mursch K, Behnke-Mursch J (2018) Polyether ether ketone cranioplasties are permeable to diagnostic ultrasound. World Neurosurg 117:142–143. https://doi.org/10.1016/j.wneu.2018.06.064
Tobias J, Hynynen K, Roemer R et al (1987) An ultrasound window to perform scanned, focused ultrasound hyperthermia treatments of brain tumors. Med Phys 14:228–234. https://doi.org/10.1118/1.596074
Giussani C, Riva M, Djonov V et al (2017) Brain ultrasound rehearsal before surgery: a pilot cadaver study. Clin Anat 30:1017–1023. https://doi.org/10.1002/ca.22919
Prada F, Del BM, Fornaro R et al (2016) Identification of residual tumor with intraoperative contrast-enhanced ultrasound during glioblastoma resection. Neurosurg Focus 40:E7. https://doi.org/10.3171/2015.11.FOCUS15573
Tranquart F, Dujardin PA, Bouché O et al (2017) Value of contrast-enhanced ultrasound quantification criteria for identifying patients not responding to bevacizumab-based therapy for colorectal liver metastases. Ultraschall der Medizin 39:544–558. https://doi.org/10.1055/s-0043-122497
Amadori M, Barone D, Scarpi E et al (2018) Dynamic contrast-enhanced ultrasonography (D-CEUS) for the early prediction of bevacizumab efficacy in patients with metastatic colorectal cancer. Eur Radiol 28:2969–2978. https://doi.org/10.1007/s00330-017-5254-5
Wolff A, Santiago GF, Belzberg M et al (2018) Adult cranioplasty reconstruction with customized cranial implants: preferred technique, timing, and biomaterials. J Craniofac Surg 29:887–894. https://doi.org/10.1097/SCS.0000000000004385
Wu SY, Aurup C, Sanchez CS et al (2018) Efficient blood-brain barrier opening in primates with neuronavigation-guided ultrasound and real-time acoustic mapping. Sci Rep 8:1–11. https://doi.org/10.1038/s41598-018-25904-9
Xu Y, Cui H, Zhu Q et al (2016) Unilateral opening of rat blood-brain barrier assisted by diagnostic ultrasound targeted microbubbles destruction. Biomed Res Int. https://doi.org/10.1155/2016/4759750
Zhao B, Chen Y, Liu J et al (2018) Blood-brain barrier disruption induced by diagnostic ultrasound combined with microbubbles in mice. Oncotarget 9:4897–4914. https://doi.org/10.18632/oncotarget.23527
Bing KF, Howlesa GP, Qib Yi, Palmeria ML, Nightingale KR (2015) Blood–Brain Barrier (BBB) disruption using a diagnostic ultrasound scanner and definity® in mice. J Investig Dermatol Ultrasound Med Bio 135:612–615. https://doi.org/10.1038/jid.2014.371
Funding
None.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by MDB, LR, GC, LS, FP, FDM. The first draft of the manuscript was written by LR and MDB and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
FP and FDM have patented the prosthesis LS, FP and FDM are stockholders of “Intelligenza Trasparente SRL”, the company which manufactures the prosthesis.
Ethical approval
The procedure was approved by the Italian Ministry of Health.
Consent to participate
Informed consent was obtained.
Consent for publication
Patient signed informed consent regarding publishing his data and photographs.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
11060_2021_3929_MOESM1_ESM.tif
Supplementary file1 (TIF 69807 KB) Supplementary Figure 1 Trans-prosthesis multiparametric US imaging is feasible. In outpatient setting, the prosthesis allows to acquire B-mode (A), CEUS (B), color doppler (C), power doppler (D), micro-V doppler (E) and fusion imaging (F) scans
Rights and permissions
About this article
Cite this article
Del Bene, M., Raspagliesi, L., Carone, G. et al. Cranial sonolucent prosthesis: a window of opportunity for neuro-oncology (and neuro-surgery). J Neurooncol 156, 529–540 (2022). https://doi.org/10.1007/s11060-021-03929-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-021-03929-x