Abstract
Introduction
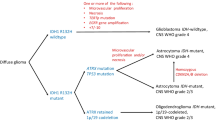
H3K27M-mutated diffuse midline gliomas (H3-DMGs) are aggressive tumors with a fatal outcome. This study integrating individual patient data (IPD) from published studies aimed to investigate the prognostic impact of different genetic alterations on survival of these patients.
Methods
We accessed PubMed and Web of Science to search for relevant articles. Studies were included if they have available data of follow-up and additional molecular investigation of H3-DMGs. For survival analysis, Kaplan–Meier analysis and Cox regression models were utilized, and corresponding hazard ratios (HR) and 95% confidence intervals (CI) were computed to analyze the impact of genetic events on overall survival (OS).
Result
We included 30 studies with 669 H3-DMGs. TP53 mutations were the most common second alteration among these neoplasms. In univariate Cox regression model, TP53 mutation was an indicator of shortened survival (HR 1.446; 95% CI 1.143–1.829) whereas ACVR1 (HR 0.712; 95% CI 0.518–0.976) and FGFR1 mutations (HR 0.408; 95% CI 0.208–0.799) conferred prolonged survival. In addition, ATRX loss was also associated with a better OS (HR 0.620; 95% CI 0.386–0.996). Adjusted for age, gender, and tumor location, the presence of TP53 mutations, the absence of ACVR1 or FGFR1 mutations remained significantly poor prognostic factors.
Conclusions
We outlined the prognostic importance of additional genetic alterations in H3-DMGs and recommended that these neoplasms should be further molecularly segregated. This may aid neuro-oncologists in appropriate risk stratification.



Similar content being viewed by others

Data availability
Not applicable.
References
Louis DN, Giannini C, Capper D, Paulus W, Figarella-Branger D, Lopes MB, Batchelor TT, Cairncross JG, van den Bent M, Wick W, Wesseling P (2018) cIMPACT-NOW update 2: diagnostic clarifications for diffuse midline glioma, H3 K27M-mutant and diffuse astrocytoma/anaplastic astrocytoma, IDH-mutant. Acta Neuropathol 135:639–642. https://doi.org/10.1007/s00401-018-1826-y
Khuong-Quang DA, Buczkowicz P, Rakopoulos P, Liu XY, Fontebasso AM, Bouffet E, Bartels U, Albrecht S, Schwartzentruber J, Letourneau L, Bourgey M, Bourque G, Montpetit A, Bourret G, Lepage P, Fleming A, Lichter P, Kool M, von Deimling A, Sturm D, Korshunov A, Faury D, Jones DT, Majewski J, Pfister SM, Jabado N, Hawkins C (2012) K27M mutation in histone H3.3 defines clinically and biologically distinct subgroups of pediatric diffuse intrinsic pontine gliomas. Acta Neuropathol 124:439–447. https://doi.org/10.1007/s00401-012-0998-0
Sturm D, Witt H, Hovestadt V, Khuong-Quang DA, Jones DT, Konermann C, Pfaff E, Tönjes M, Sill M, Bender S, Kool M, Zapatka M, Becker N, Zucknick M, Hielscher T, Liu XY, Fontebasso AM, Ryzhova M, Albrecht S, Jacob K, Wolter M, Ebinger M, Schuhmann MU, van Meter T, Frühwald MC, Hauch H, Pekrun A, Radlwimmer B, Niehues T, von Komorowski G, Dürken M, Kulozik AE, Madden J, Donson A, Foreman NK, Drissi R, Fouladi M, Scheurlen W, von Deimling A, Monoranu C, Roggendorf W, Herold-Mende C, Unterberg A, Kramm CM, Felsberg J, Hartmann C, Wiestler B, Wick W, Milde T, Witt O, Lindroth AM, Schwartzentruber J, Faury D, Fleming A, Zakrzewska M, Liberski PP, Zakrzewski K, Hauser P, Garami M, Klekner A, Bognar L, Morrissy S, Cavalli F, Taylor MD, van Sluis P, Koster J, Versteeg R, Volckmann R, Mikkelsen T, Aldape K, Reifenberger G, Collins VP, Majewski J, Korshunov A, Lichter P, Plass C, Jabado N, Pfister SM (2012) Hotspot mutations in H3F3A and IDH1 define distinct epigenetic and biological subgroups of glioblastoma. Cancer Cell 22:425–437. https://doi.org/10.1016/j.ccr.2012.08.024
Ray-Gallet D, Woolfe A, Vassias I, Pellentz C, Lacoste N, Puri A, Schultz DC, Pchelintsev NA, Adams PD, Jansen LE, Almouzni G (2011) Dynamics of histone H3 deposition in vivo reveal a nucleosome gap-filling mechanism for H3.3 to maintain chromatin integrity. Mol Cell 44:928–941. https://doi.org/10.1016/j.molcel.2011.12.006
Yuen BT, Knoepfler PS (2013) Histone H3.3 mutations: a variant path to cancer. Cancer Cell 24:567–574. https://doi.org/10.1016/j.ccr.2013.09.015
Tagami H, Ray-Gallet D, Almouzni G, Nakatani Y (2004) Histone H3.1 and H3.3 complexes mediate nucleosome assembly pathways dependent or independent of DNA synthesis. Cell 116:51–61. https://doi.org/10.1016/s0092-8674(03)01064-x
Mackay A, Burford A, Molinari V, Jones DTW, Izquierdo E, Brouwer-Visser J, Giangaspero F, Haberler C, Pietsch T, Jacques TS, Figarella-Branger D, Rodriguez D, Morgan PS, Raman P, Waanders AJ, Resnick AC, Massimino M, Garrè ML, Smith H, Capper D, Pfister SM, Würdinger T, Tam R, Garcia J, Thakur MD, Vassal G, Grill J, Jaspan T, Varlet P, Jones C (2018) Molecular, pathological, radiological, and immune profiling of non-brainstem pediatric high-grade glioma from the HERBY Phase II randomized trial. Cancer Cell 33:829-842.e825. https://doi.org/10.1016/j.ccell.2018.04.004
Castel D, Philippe C, Calmon R, Le Dret L, Truffaux N, Boddaert N, Pagès M, Taylor KR, Saulnier P, Lacroix L, Mackay A, Jones C, Sainte-Rose C, Blauwblomme T, Andreiuolo F, Puget S, Grill J, Varlet P, Debily MA (2015) Histone H3F3A and HIST1H3B K27M mutations define two subgroups of diffuse intrinsic pontine gliomas with different prognosis and phenotypes. Acta Neuropathol 130:815–827. https://doi.org/10.1007/s00401-015-1478-0
Vuong HG, Tran TTK, Ngo HTT, Pham TQ, Nakazawa T, Fung KM, Hassell L, Katoh R, Kondo T (2019) Prognostic significance of genetic biomarkers in isocitrate dehydrogenase-wild-type lower-grade glioma: the need to further stratify this tumor entity—a meta-analysis. Eur J Neurol 26:379–387. https://doi.org/10.1111/ene.13826
Aibaidula A, Chan AK, Shi Z, Li Y, Zhang R, Yang R, Li KK, Chung NY, Yao Y, Zhou L, Wu J, Chen H, Ng HK (2017) Adult IDH wild-type lower-grade gliomas should be further stratified. Neuro Oncol 19:1327–1337. https://doi.org/10.1093/neuonc/nox078
Korshunov A, Ryzhova M, Hovestadt V, Bender S, Sturm D, Capper D, Meyer J, Schrimpf D, Kool M, Northcott PA, Zheludkova O, Milde T, Witt O, Kulozik AE, Reifenberger G, Jabado N, Perry A, Lichter P, von Deimling A, Pfister SM, Jones DT (2015) Integrated analysis of pediatric glioblastoma reveals a subset of biologically favorable tumors with associated molecular prognostic markers. Acta Neuropathol 129:669–678. https://doi.org/10.1007/s00401-015-1405-4
Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6:e1000097. https://doi.org/10.1371/journal.pmed.1000097
Aihara K, Mukasa A, Gotoh K, Saito K, Nagae G, Tsuji S, Tatsuno K, Yamamoto S, Takayanagi S, Narita Y, Shibui S, Aburatani H, Saito N (2014) H3F3A K27M mutations in thalamic gliomas from young adult patients. Neuro Oncol 16:140–146. https://doi.org/10.1093/neuonc/not144
Alvi MA, Ida CM, Paolini MA, Kerezoudis P, Meyer J, Barr Fritcher EG, Goncalves S, Meyer FB, Bydon M, Raghunathan A (2019) Spinal cord high-grade infiltrating gliomas in adults: clinico-pathological and molecular evaluation. Modern Pathol 32: 1236–1243 https://doi.org/10.1038/s41379-019-0271-3
Bruzek AK, Ravi K, Muruganand A, Wadden J, Babila CM, Cantor E, Tunkle L, Wierzbicki K, Stallard S, Dickson RP, Wolfe I, Mody R, Schwartz J, Franson A, Robertson PL, Muraszko KM, Maher CO, Garton HJL, Qin T, Koschmann C (2020) Electronic DNA analysis of CSF cell-free tumor DNA to quantify multi-gene molecular response in pediatric high-grade glioma. Clin Cancer Res 26:6266–6276. https://doi.org/10.1158/1078-0432.ccr-20-2066
Buczkowicz P, Hoeman C, Rakopoulos P, Pajovic S, Letourneau L, Dzamba M, Morrison A, Lewis P, Bouffet E, Bartels U, Zuccaro J, Agnihotri S, Ryall S, Barszczyk M, Chornenkyy Y, Bourgey M, Bourque G, Montpetit A, Cordero F, Castelo-Branco P, Mangerel J, Tabori U, Ho KC, Huang A, Taylor KR, Mackay A, Bendel AE, Nazarian J, Fangusaro JR, Karajannis MA, Zagzag D, Foreman NK, Donson A, Hegert JV, Smith A, Chan J, Lafay-Cousin L, Dunn S, Hukin J, Dunham C, Scheinemann K, Michaud J, Zelcer S, Ramsay D, Cain J, Brennan C, Souweidane MM, Jones C, Allis CD, Brudno M, Becher O, Hawkins C (2014) Genomic analysis of diffuse intrinsic pontine gliomas identifies three molecular subgroups and recurrent activating ACVR1 mutations. Nat Genet 46:451–456. https://doi.org/10.1038/ng.2936
Daoud EV, Rajaram V, Cai C, Oberle RJ, Martin GR, Raisanen JM, White CL 3rd, Foong C, Mickey BE, Pan E, Hatanpaa KJ (2018) Adult brainstem gliomas with H3K27M mutation: radiology, pathology, and prognosis. J Neuropathol Exp Neurol 77:302–311. https://doi.org/10.1093/jnen/nly006
Dono A, Takayasu T, Ballester LY, Esquenazi Y (2020) Adult diffuse midline gliomas: clinical, radiological, and genetic characteristics. J Clin Neurosci 82:1–8. https://doi.org/10.1016/j.jocn.2020.10.005
Dufour C, Perbet R, Leblond P, Vasseur R, Stechly L, Pierache A, Reyns N, Touzet G, Le Rhun E, Vinchon M, Maurage CA, Escande F, Renaud F (2020) Identification of prognostic markers in diffuse midline gliomas H3K27M-mutant. Brain Pathol (Zurich, Switzerland) 30:179–190. https://doi.org/10.1111/bpa.12768
Eschbacher KL, Ida CM, Johnson DR, Alvi MA, Jenkins SM, Ruff MW, Kerezoudis P, Neth BJ, Pasion RM, Daniels DJ, Kizilbash SH, Raghunathan A (2021) Diffuse gliomas of the brainstem and cerebellum in adults show molecular heterogeneity. Am J Surg Pathol. https://doi.org/10.1097/pas.0000000000001690
Garibotto F, Madia F, Milanaccio C, Verrico A, Piccardo A, Tortora D, Piatelli G, Diana MC, Capra V, Garrè ML, Rossi A, Morana G (2020) Pediatric diffuse midline gliomas H3 K27M-mutant and non-histone mutant midline high-grade gliomas in neurofibromatosis type 1 in comparison with non-syndromic children: a single-center pilot study. Front Oncol 10:795. https://doi.org/10.3389/fonc.2020.00795
Gojo J, Pavelka Z, Zapletalova D, Schmook MT, Mayr L, Madlener S, Kyr M, Vejmelkova K, Smrcka M, Czech T, Dorfer C, Skotakova J, Azizi AA, Chocholous M, Reisinger D, Lastovicka D, Valik D, Haberler C, Peyrl A, Noskova H, Pál K, Jezova M, Veselska R, Kozakova S, Slaby O, Slavc I, Sterba J (2019) Personalized treatment of H3K27M-mutant pediatric diffuse gliomas provides improved therapeutic opportunities. Front Oncol 9:1436. https://doi.org/10.3389/fonc.2019.01436
Hoffman LM, DeWire M, Ryall S, Buczkowicz P, Leach J, Miles L, Ramani A, Brudno M, Kumar SS, Drissi R, Dexheimer P, Salloum R, Chow L, Hummel T, Stevenson C, Lu QR, Jones B, Witte D, Aronow B, Hawkins CE, Fouladi M (2016) Spatial genomic heterogeneity in diffuse intrinsic pontine and midline high-grade glioma: implications for diagnostic biopsy and targeted therapeutics. Acta Neuropathol Commun. https://doi.org/10.1186/s40478-015-0269-0
Karlowee V, Amatya VJ, Takayasu T, Takano M, Yonezawa U, Takeshima Y, Sugiyama K, Kurisu K, Yamasaki F (2019) Immunostaining of increased expression of enhancer of Zeste Homolog 2 (EZH2) in diffuse midline glioma H3K27M-mutant patients with poor survival. Pathobiology 86:152–161. https://doi.org/10.1159/000496691
Mackay A, Burford A, Carvalho D, Izquierdo E, Fazal-Salom J, Taylor KR, Bjerke L, Clarke M, Vinci M, Nandhabalan M, Temelso S, Popov S, Molinari V, Raman P, Waanders AJ, Han HJ, Gupta S, Marshall L, Zacharoulis S, Vaidya S, Mandeville HC, Bridges LR, Martin AJ, Al-Sarraj S, Chandler C, Ng HK, Li X, Mu K, Trabelsi S, Brahim DH, Kisljakov AN, Konovalov DM, Moore AS, Carcaboso AM, Sunol M, de Torres C, Cruz O, Mora J, Shats LI, Stavale JN, Bidinotto LT, Reis RM, Entz-Werle N, Farrell M, Cryan J, Crimmins D, Caird J, Pears J, Monje M, Debily MA, Castel D, Grill J, Hawkins C, Nikbakht H, Jabado N, Baker SJ, Pfister SM, Jones DTW, Fouladi M, von Bueren AO, Baudis M, Resnick A, Jones C (2017) Integrated molecular meta-analysis of 1,000 pediatric high-grade and diffuse intrinsic pontine glioma. Cancer Cell 32:520-537.e525. https://doi.org/10.1016/j.ccell.2017.08.017
Meyronet D, Esteban-Mader M, Bonnet C, Joly MO, Uro-Coste E, Amiel-Benouaich A, Forest F, Rousselot-Denis C, Burel-Vandenbos F, Bourg V, Guyotat J, Fenouil T, Jouvet A, Honnorat J, Ducray F (2017) Characteristics of H3 K27M-mutant gliomas in adults. Neuro Oncol 19:1127–1134. https://doi.org/10.1093/neuonc/now274
Mueller S, Jain P, Liang WS, Kilburn L, Kline C, Gupta N, Panditharatna E, Magge SN, Zhang B, Zhu Y, Crawford JR, Banerjee A, Nazemi K, Packer RJ, Petritsch CK, Truffaux N, Roos A, Nasser S, Phillips JJ, Solomon D, Molinaro A, Waanders AJ, Byron SA, Berens ME, Kuhn J, Nazarian J, Prados M, Resnick AC (2019) A pilot precision medicine trial for children with diffuse intrinsic pontine glioma-PNOC003: a report from the Pacific Pediatric Neuro-Oncology Consortium. Int J Cancer 145:1889–1901. https://doi.org/10.1002/ijc.32258
Nomura M, Mukasa A, Nagae G, Yamamoto S, Tatsuno K, Ueda H, Fukuda S, Umeda T, Suzuki T, Otani R, Kobayashi K, Maruyama T, Tanaka S, Takayanagi S, Nejo T, Takahashi S, Ichimura K, Nakamura T, Muragaki Y, Narita Y, Nagane M, Ueki K, Nishikawa R, Shibahara J, Aburatani H, Saito N (2017) Distinct molecular profile of diffuse cerebellar gliomas. Acta Neuropathol 134:941–956. https://doi.org/10.1007/s00401-017-1771-1
Pan C, Diplas BH, Chen X, Wu Y, Xiao X, Jiang L, Geng Y, Xu C, Sun Y, Zhang P, Wu W, Wang Y, Wu Z, Zhang J, Jiao Y, Yan H, Zhang L (2019) Molecular profiling of tumors of the brainstem by sequencing of CSF-derived circulating tumor DNA. Acta Neuropathol 137:297–306. https://doi.org/10.1007/s00401-018-1936-6
Picca A, Berzero G, Bielle F, Touat M, Savatovsky J, Polivka M, Trisolini E, Meunier S, Schmitt Y, Idbaih A, Hoang-Xuan K, Delattre JY, Mokhtari K, Di Stefano AL, Sanson M (2018) FGFR1 actionable mutations, molecular specificities, and outcome of adult midline gliomas. Neurology 90:e2086–e2094. https://doi.org/10.1212/wnl.0000000000005658
Ryall S, Krishnatry R, Arnoldo A, Buczkowicz P, Mistry M, Siddaway R, Ling C, Pajovic S, Yu M, Rubin JB, Hukin J, Steinbok P, Bartels U, Bouffet E, Tabori U, Hawkins C (2016) Targeted detection of genetic alterations reveal the prognostic impact of H3K27M and MAPK pathway aberrations in paediatric thalamic glioma. Acta Neuropathol Commun 4:93. https://doi.org/10.1186/s40478-016-0353-0
Schwartzentruber J, Korshunov A, Liu XY, Jones DT, Pfaff E, Jacob K, Sturm D, Fontebasso AM, Quang DA, Tönjes M, Hovestadt V, Albrecht S, Kool M, Nantel A, Konermann C, Lindroth A, Jäger N, Rausch T, Ryzhova M, Korbel JO, Hielscher T, Hauser P, Garami M, Klekner A, Bognar L, Ebinger M, Schuhmann MU, Scheurlen W, Pekrun A, Frühwald MC, Roggendorf W, Kramm C, Dürken M, Atkinson J, Lepage P, Montpetit A, Zakrzewska M, Zakrzewski K, Liberski PP, Dong Z, Siegel P, Kulozik AE, Zapatka M, Guha A, Malkin D, Felsberg J, Reifenberger G, von Deimling A, Ichimura K, Collins VP, Witt H, Milde T, Witt O, Zhang C, Castelo-Branco P, Lichter P, Faury D, Tabori U, Plass C, Majewski J, Pfister SM, Jabado N (2012) Driver mutations in histone H3.3 and chromatin remodelling genes in paediatric glioblastoma. Nature 482:226–231. https://doi.org/10.1038/nature10833
Taylor KR, Mackay A, Truffaux N, Butterfield Y, Morozova O, Philippe C, Castel D, Grasso CS, Vinci M, Carvalho D, Carcaboso AM, de Torres C, Cruz O, Mora J, Entz-Werle N, Ingram WJ, Monje M, Hargrave D, Bullock AN, Puget S, Yip S, Jones C, Grill J (2014) Recurrent activating ACVR1 mutations in diffuse intrinsic pontine glioma. Nat Genet 46:457–461. https://doi.org/10.1038/ng.2925
Wang L, Li Z, Zhang M, Piao Y, Chen L, Liang H, Wei Y, Hu Z, Zhao L, Teng L, Lu D (2018) H3 K27M-mutant diffuse midline gliomas in different anatomical locations. Hum Pathol 78:89–96. https://doi.org/10.1016/j.humpath.2018.04.015
Wang Y, Feng LL, Ji PG, Liu JH, Guo SC, Zhai YL, Sankey EW, Wang Y, Xue YR, Wang N, Lou M, Xu M, Chao M, Gao GD, Qu Y, Gong L, Wang L (2020) Clinical features and molecular markers on diffuse midline gliomas with H3K27M mutations: a 43 cases retrospective cohort study. Front Oncol 10:602553. https://doi.org/10.3389/fonc.2020.602553
Wu G, Diaz AK, Paugh BS, Rankin SL, Ju B, Li Y, Zhu X, Qu C, Chen X, Zhang J, Easton J, Edmonson M, Ma X, Lu C, Nagahawatte P, Hedlund E, Rusch M, Pounds S, Lin T, Onar-Thomas A, Huether R, Kriwacki R, Parker M, Gupta P, Becksfort J, Wei L, Mulder HL, Boggs K, Vadodaria B, Yergeau D, Russell JC, Ochoa K, Fulton RS, Fulton LL, Jones C, Boop FA, Broniscer A, Wetmore C, Gajjar A, Ding L, Mardis ER, Wilson RK, Taylor MR, Downing JR, Ellison DW, Zhang J, Baker SJ, the St. Jude Children’s Research Hospital-Washington University Pediatric Cancer Genome P (2014) The genomic landscape of diffuse intrinsic pontine glioma and pediatric non-brainstem high-grade glioma. Nat Genet 46:444–450. https://doi.org/10.1038/ng.2938
Yi S, Choi S, Shin DA, Kim DS, Choi J, Ha Y, Kim KN, Suh CO, Chang JH, Kim SH, Yoon DH (2019) Impact of H3.3 K27M mutation on prognosis and survival of Grade IV spinal cord glioma on the basis of new 2016 world health organization classification of the central nervous system. Neurosurgery 84:1072–1081. https://doi.org/10.1093/neuros/nyy150
Zhou C, Zhao H, Yang F, Huangfu L, Dong C, Wang S, Zhang J (2021) Clinical and genetic features of brainstem glioma in adults: a report of 50 cases in a single center. J Clin Neurol (Seoul, Korea) 17:220–228. https://doi.org/10.3988/jcn.2021.17.2.220
Buczkowicz P, Bartels U, Bouffet E, Becher O, Hawkins C (2014) Histopathological spectrum of paediatric diffuse intrinsic pontine glioma: diagnostic and therapeutic implications. Acta Neuropathol 128:573–581. https://doi.org/10.1007/s00401-014-1319-6
Mosaab A, El-Ayadi M, Khorshed EN, Amer N, Refaat A, El-Beltagy M, Hassan Z, Soror SH, Zaghloul MS, El-Naggar S (2020) Histone H3K27M mutation overrides histological grading in pediatric gliomas. Sci Rep 10:8368. https://doi.org/10.1038/s41598-020-65272-x
Karremann M, Gielen GH, Hoffmann M, Wiese M, Colditz N, Warmuth-Metz M, Bison B, Claviez A, van Vuurden DG, von Bueren AO, Gessi M, Kühnle I, Hans VH, Benesch M, Sturm D, Kortmann RD, Waha A, Pietsch T, Kramm CM (2018) Diffuse high-grade gliomas with H3 K27M mutations carry a dismal prognosis independent of tumor location. Neuro Oncol 20:123–131. https://doi.org/10.1093/neuonc/nox149
Castel D, Philippe C, Kergrohen T, Sill M, Merlevede J, Barret E, Puget S, Sainte-Rose C, Kramm CM, Jones C, Varlet P, Pfister SM, Grill J, Jones DTW, Debily MA (2018) Transcriptomic and epigenetic profiling of “diffuse midline gliomas, H3 K27M-mutant” discriminate two subgroups based on the type of histone H3 mutated and not supratentorial or infratentorial location. Acta Neuropathol Commun 6:117. https://doi.org/10.1186/s40478-018-0614-1
Schüller U, Iglauer P, Dorostkar MM, Mawrin C, Herms J, Giese A, Glatzel M, Neumann JE (2021) Mutations within FGFR1 are associated with superior outcome in a series of 83 diffuse midline gliomas with H3F3A K27M mutations. Acta Neuropathol 141:323–325. https://doi.org/10.1007/s00401-020-02259-y
Bögler O, Huang HJ, Kleihues P, Cavenee WK (1995) The p53 gene and its role in human brain tumors. Glia 15:308–327. https://doi.org/10.1002/glia.440150311
Fontebasso AM, Schwartzentruber J, Khuong-Quang DA, Liu XY, Sturm D, Korshunov A, Jones DT, Witt H, Kool M, Albrecht S, Fleming A, Hadjadj D, Busche S, Lepage P, Montpetit A, Staffa A, Gerges N, Zakrzewska M, Zakrzewski K, Liberski PP, Hauser P, Garami M, Klekner A, Bognar L, Zadeh G, Faury D, Pfister SM, Jabado N, Majewski J (2013) Mutations in SETD2 and genes affecting histone H3K36 methylation target hemispheric high-grade gliomas. Acta Neuropathol 125:659–669. https://doi.org/10.1007/s00401-013-1095-8
Nikbakht H, Panditharatna E, Mikael LG, Li R, Gayden T, Osmond M, Ho CY, Kambhampati M, Hwang EI, Faury D, Siu A, Papillon-Cavanagh S, Bechet D, Ligon KL, Ellezam B, Ingram WJ, Stinson C, Moore AS, Warren KE, Karamchandani J, Packer RJ, Jabado N, Majewski J, Nazarian J (2016) Spatial and temporal homogeneity of driver mutations in diffuse intrinsic pontine glioma. Nat Commun 7:11185. https://doi.org/10.1038/ncomms11185
Werbrouck C, Evangelista CCS, Lobón-Iglesias MJ, Barret E, Le Teuff G, Merlevede J, Brusini R, Kergrohen T, Mondini M, Bolle S, Varlet P, Beccaria K, Boddaert N, Puget S, Grill J, Debily MA, Castel D (2019) TP53 pathway alterations drive radioresistance in diffuse intrinsic pontine gliomas (DIPG). Clin Cancer Res 25:6788–6800. https://doi.org/10.1158/1078-0432.ccr-19-0126
Wiestler B, Capper D, Holland-Letz T, Korshunov A, von Deimling A, Pfister SM, Platten M, Weller M, Wick W (2013) ATRX loss refines the classification of anaplastic gliomas and identifies a subgroup of IDH mutant astrocytic tumors with better prognosis. Acta Neuropathol 126:443–451. https://doi.org/10.1007/s00401-013-1156-z
Vuong HG, Altibi AMA, Duong UNP, Ngo HTT, Pham TQ, Fung KM, Hassell L (2018) BRAF mutation is associated with an improved survival in glioma-a systematic review and meta-analysis. Mol Neurobiol 55:3718–3724. https://doi.org/10.1007/s12035-017-0599-y
Mistry M, Zhukova N, Merico D, Rakopoulos P, Krishnatry R, Shago M, Stavropoulos J, Alon N, Pole JD, Ray PN, Navickiene V, Mangerel J, Remke M, Buczkowicz P, Ramaswamy V, Guerreiro Stucklin A, Li M, Young EJ, Zhang C, Castelo-Branco P, Bakry D, Laughlin S, Shlien A, Chan J, Ligon KL, Rutka JT, Dirks PB, Taylor MD, Greenberg M, Malkin D, Huang A, Bouffet E, Hawkins CE, Tabori U (2015) BRAF mutation and CDKN2A deletion define a clinically distinct subgroup of childhood secondary high-grade glioma. J Clin Oncol 33:1015–1022. https://doi.org/10.1200/jco.2014.58.3922
Vuong HG, Altibi AMA, Duong UNP, Ngo HTT, Pham TQ, Chan AK, Park CK, Fung KM, Hassell L (2017) TERT promoter mutation and its interaction with IDH mutations in glioma: combined TERT promoter and IDH mutations stratifies lower-grade glioma into distinct survival subgroups-a meta-analysis of aggregate data. Crit Rev Oncol Hematol 120:1–9. https://doi.org/10.1016/j.critrevonc.2017.09.013
Smith JS, Tachibana I, Passe SM, Huntley BK, Borell TJ, Iturria N, O'Fallon JR, Schaefer PL, Scheithauer BW, James CD, Buckner JC, Jenkins RB (2001) PTEN mutation, EGFR amplification, and outcome in patients with anaplastic astrocytoma and glioblastoma multiforme. JNCI 93: 1246–1256. https://doi.org/10.1093/jnci/93.16.1246
Vuong HG, Nguyen TQ, Ngo TNM, Nguyen HC, Fung KM, Dunn IF (2020) The interaction between TERT promoter mutation and MGMT promoter methylation on overall survival of glioma patients: a meta-analysis. BMC Cancer 20:897. https://doi.org/10.1186/s12885-020-07364-5
Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D, Hawkins C, Ng HK, Pfister SM, Reifenberger G, Soffietti R, von Deimling A, Ellison DW (2021) The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro Oncol 23:1231–1251. https://doi.org/10.1093/neuonc/noab106
Acknowledgements
Not applicable.
Funding
This study receives no funding support.
Author information
Authors and Affiliations
Contributions
HGV: conceptualization, data curation, formal analysis, investigation, methodology, project administration, software, validation, writing-review, and editing. HTL: data curation, formal analysis, investigation, methodology, writing-review. TNMN: data curation, formal analysis, investigation, methodology, writing-review. KMF: data curation, formal analysis, investigation, methodology, project administration, validation, writing-review, and editing. JDB: data curation, formal analysis, investigation, methodology, project administration, validation, writing-review, and editing. RMK: data curation, formal analysis, investigation, methodology, project administration, validation, writing-review, and editing. IFD: data curation, formal analysis, investigation, methodology, project administration, validation, writing-review, editing, and supervision. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Ethical approval
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Vuong, H.G., Le, H.T., Ngo, T.N.M. et al. H3K27M-mutant diffuse midline gliomas should be further molecularly stratified: an integrated analysis of 669 patients. J Neurooncol 155, 225–234 (2021). https://doi.org/10.1007/s11060-021-03890-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-021-03890-9