Abstract
Introduction
Combination therapy for melanoma brain metastases (MM) using stereotactic radiosurgery (SRS) and immune checkpoint-inhibition (ICI) or targeted therapy (TT) is currently of high interest. In this collective, time evolution and incidence of imaging findings indicative of pseudoprogression is sparsely researched. We therefore investigated time-course of MRI characteristics in these patients.
Methods
Data were obtained retrospectively from 27 patients (12 female, 15 male; mean 61 years, total of 169 MMs). Single lesion volumes, total MM burden and edema volumes were analyzed at baseline and follow-up MRIs in 2 months intervals after SRS up to 24 months. The occurrence of intralesional hemorrhages was recorded.
Results
17 patients (80 MM) received ICI, 8 (62 MM) TT and 2 (27 MM) ICI + TT concomitantly to SRS. MM-localization was frontal (n = 89), temporal (n = 23), parietal (n = 20), occipital (n = 10), basal ganglia/thalamus/insula (n = 10) and cerebellar (n = 10). A volumetric progression of MM 2–4 months after SRS was observed in combined treatment with ICI (p = 0.028) and ICI + TT (p = 0.043), whereas MMs treated with TT showed an early volumetric regression (p = 0.004). Edema volumes moderately correlated with total MM volumes (r = 0.57; p < 0.0001). Volumetric behavior did not differ significantly over time regarding lesions’ initial sizes or localizations. No significant differences between groups were observed regarding rates of post-SRS intralesional hemorrhages.
Conclusion
Reversible volumetric increases in terms of pseudoprogression are observed 2–4 months after SRS in patients with MM concomitantly treated with ICI and ICI + TT, rarely after TT. Edema volumes mirror total MM volumes. Medical treatment type does not significantly affect rates of intralesional hemorrhage.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Brain metastases are common in patients with melanoma. About 20% of patients with newly diagnosed melanoma have brain metastases, and in advanced metastatic melanoma, brain metastases are found in 44% of patients [1]. Overall median survival in these patients used to be just 4 months [2]. Hitherto existing therapeutic options were limited. Systemic chemotherapy is presumed to be ineffective due to low local drug concentrations owing to the blood–brain barrier [1]. Effective local treatments include surgery and radiosurgery for patients with a limited number of metastases [3, 4]. In contrast, the evidence for whole brain irradiation for disseminated disease remains inconclusive [5].
As treatment options for melanoma brain metastases remain challenging, they gained new impetus with the introduction of targeted therapies (TT) and immune checkpoint inhibitors (ICI). These include immunotherapy with interleukin-2, antibodies targeting programmed cell death protein 1 (PD-1) [Nivolumab, Pembrolizumab], antibodies targeting cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) [Ipilimumab] and inhibition of the mitogen-activated protein (MAP) kinase pathway in melanomas with a V600-mutation using BRAF- [Vemurafenib, Dabrafenib, Encorafenib] or MEK-inhibitors [Cobimetinib, Trametinib and Binimetinib] [6].
Studies that investigated the combination of radiosurgery and TT or ICI in patients with melanoma brain metastases (MM) showed an improved survival over TT and ICI alone with median overall survival times ranging from 10.9 to 15.1 months [7,8,9,10].
To monitor treatment and detect associated complications in these patients, serial follow-up using magnetic resonance imaging (MRI) is performed. However, in patients undergoing radiosurgery and TT or ICI therapy it remains challenging as MRI findings are often difficult to interpret. Both immunomodulatory drug-induced mechanisms and radiation-induced changes may contribute to imaging findings that may simulate progressive disease [11, 12].
The aim of our study was to retrospectively investigate time course, time evolution and regression of MM as well as frequency of findings indicative of pseudoprogression in patients undergoing combined treatment with CyberKnife radiosurgery and ICI or TT.
Material and methods
Patient data
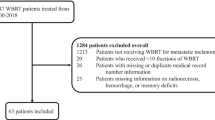
Data from patients with MM treated with SRS and immunotherapy were collected retrospectively from an institutional database. We included patients with MM that were treated with CyberKnife single-fraction robotic SRS between 2013 and 2017. Ethics approval was obtained by the ethics committee of the Johann Wolfgang Goethe-University Frankfurt. In total, 27 patients with 169 lesions were analyzed. Data collected included baseline demographics, UICC stage, BRAF (V-raf murine sarcoma viral oncogene homolog B1) and NRAS (neuroblastoma rat sarcoma oncogene) mutational status, number and localization of MM at the planning MRI scan, prior treatments, ICI type and cycles as well as TT type and cycles. Imaging was performed for treatment planning before SRS and at 2 months intervals thereafter. Follow-up period was 24 months.
CyberKnife radiosurgery
Single session radiosurgery treatments were performed using the CyberKnife VSI Radiosurgery System (Accuray, Sunnyvale, USA). This system consists of a 6 MV linear accelerator mounted a computer-controlled robotic arm. Patient immobilization is achieved by a thermoplastic mask that is fixed to the treatment table. Single fractions ranged from 18 to 22 Gy (median 18 Gy). 21 patients received dexamethasone (4–8 mg) on the day of treatment to prevent from sudden brain edema.
Immunotherapy
ICI were administered with at least three cycles with ipilimumab given intravenously at 3 mg/kg every 3 weeks, nivolumab at 3 mg/kg every 2 weeks and pembrolizumab at 2 mg/kg every 3 weeks. TT were administered on a daily basis with vemurafenib 240–960 mg/d, cobimetinib 20 mg/d, dabrafenib 300 mg/d and trametinib 2 mg/d.
MR imaging
All scans were performed on a 1.5 T MRI system (Achieva 1.5 T, Philips Health Systems, Eindhoven, The Netherlands) using a 15-channel phased-array head coil. Images of the whole brain were acquired including the following sequences:
-
1.
T1-weighted axial fast spin-echo (time repetition [TR] 664 ms, time echo [TE] 14 ms, slice thickness 5 mm, gap 0.5 mm, matrix 512 × 512, field of view [FOV] 230 mm2).
-
2.
T2-weighted axial fast spin-echo (TR 8,000 ms, TE 120 ms, slice thickness 2.0 mm, spacing 2.2 mm, matrix 560 × 560, FOV 260 mm2).
-
3.
T2*-weighted axial (TR 520 ms, TE 14 ms, slice thickness 5 mm, gap 5.5 mm, matrix 320 × 320, FOV 230 mm2).
-
4.
3D T1-weighted axial (TR 25 ms, TE 1.9 ms, flip angle 30°, section thickness 1.5 mm, matrix 256 × 256, FOV 210 mm2) after intravenous administration of single-dose gadolinium contrast agent (Gadovist, Bayer Vital, Leverkusen, Germany; 0.1 mmol/kg).
Volumetric analyses
Lesions’ volumes were ascertained in isotropic T1-weighted contrast enhanced images using a semiautomatic edge detection tool (IntelliSpace Portal 11, Philips, Eindhoven, The Netherlands). Volumes of edemas were analyzed by volumetry of perilesional hyperintensities in T2-weighted images using the same software. Delimitable lesions with diameters < 1 mm were considered immeasurable in terms of measuring inaccuracy. The presence or absence of metastatic hemorrhages was ascertained by the presence of newly emerging hyperintensities on unenhanced T1-weighted and/or hypointensities on T2*-weighted images.
Statistical analysis
Statistical analysis was performed using GraphPad Prism version 6 (GraphPad Software, San Diego, USA). Values are reported as the mean and standard deviation unless otherwise specified. Normal data distribution was ascertained using the D’Agostino-Pearson omnibus normality test. A paired Student’s t test was performed for the comparison of pre- and postoperative imaging series. An unpaired Student’s t test was used for all other comparisons. The Mann–Whitney rank-sum test was used for the comparison of non-normal distributed data, the Kruskal–Wallis-test for comparison of nominal scaled data. A p value < 0.05 was considered as statistically significant.
Results
27 patients with a total of 169 MM (1–26 lesions per patient, mean 5.7) were identified. Patient data is summarized in Table 1.
17 patients harboring 80 lesions received ICI, 8 patients with 62 lesions received TT and 2 patients with 27 lesions were treated with a combination of ICI and TT concomitant to SRS. In 3 patients that received ICI, 1 patient treated with TT, and 1 patient that received ICI + TT, medical therapy started 3–4 months preceding SRS, in all other patients, concurrent therapy with ICI and/or TT was administered within ± 4 weeks of SRS procedure.
Mean patient age at the time of SRS was 61 years. There were no significant differences regarding age between the groups. SRS planning target volume (PTV) doses per lesion did not differ significantly between patients that received ICI (median 20 Gy), TT (median 19 Gy) and ICI + TT (median 19 Gy). Patients had different antecedent treatments (> 6 months preceding SRS) including systemic chemotherapy (n = 3), interferone treatment (n = 9), dendritic cell-based vaccination (n = 1), and surgical resection of metastases outside the central nervous system (n = 15).
There were no significant differences between the groups regarding UICC stage (p = 0.49) and time from MM diagnosis to SRS (p = 0.60).
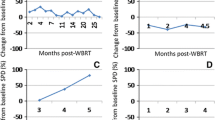
After SRS, in patients treated with ICI, an early and significant (p = 0.028) increase in lesional volumes was detectable at first follow-up at 2 months (Fig. 1).
In this group, there were no significant differences between PD-1 and CTLA-4 ICI (p = 0.069). A later regression was seen at 4 months after SRS, with MM volumes not significantly different from baseline values.
In patients treated with ICI + TT, a significant volumetric increase of MM at 2 months (p = 0.043) and 4 months (p = 0.037) after SRS occurred, compared to baseline values, respectively. In patients treated with TT, MM showed an early volumetric regression (p = 0.004) (Fig. 1). Regarding individual MMs, in first MRI follow up 2 months after SRS, an increase in lesional volumes was seen in 25/80 MM (31.3%) treated with ICI, in 12/27 MM (44.4%) treated with ICI + TT, but only in 9/62 MM (14.5%) treated with TT.
There were no significant differences in volumetric response after SRS regarding lesion localization and initial MM volumes.
Total edema volumes moderately correlated (r = 0.57; p < 0.0001) with total MM volumes for any patient at given follow-up time points (Fig. 2).
In patients treated with ICI + TT, 23/27 (85.2%) metastases showed an increase in volumes and perifocal edema at 12 months follow-up after SRS compared with nadir at 8 months. Of these 10/23 (43.5%) showed an early reversible increase within the first 4 months.
In patients treated with TT, 50/62 (80.6%) metastases had an increase in volumes at 12 months follow-up after SRS compared with nadir at 8 months. In this group, none of these metastases showed early reversible volume increases.
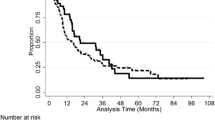
Median overall survival from the date of SRS was 13 months. In patients receiving ICI + TT and TT, median survival was 17 and 11.5 months (p = n.s.), respectively. Median survival was not reached in patients treated with ICI within the 24 months follow-up period. Plots of overall survival and survival depending on medication are shown in Fig. 3.
12 patients (44.4%) were positive for BRAF mutation and 4 (14.8%) positive for NRAS mutation. MMs with BRAF positive status showed an early volumetric regression after SRS (p = 0.038) compared to those with BRAF negative status (Fig. 4a). 4/12 (33.3%) BRAF positive patients were treated with ICI, 2/12 (16.7%) with ICI + TT and 6/12 (50%) with TT, respectively.
Up to 6 months after SRS, there were no significant differences in MM volumes between NRAS positive and NRAS negative patients (Fig. 4b).
10/169 MMs (= 5.9%) showed preexisting bleeds. Newly emerging metastatic bleeds in first follow-up after SRS occurred in 37/169 MMs (= 21.9%). There was no significant difference for any given group. In one case, bleeding led to a significant mass effect, necessitating surgical intervention. Bleeding with a longer interval to SRS occurred in 4 metastases at 3, 6, 8 and 24 months follow-up, respectively.
Discussion
The aim of our study was to determine time evolution and incidence of MRI findings indicative of pseudoprogression in the monitoring of MM treated with CyberKnife SRS and concomitant ICI or TT.
In general, SRS is considered as the primary form of radiation therapy for patients with a limited number of small to medium-sized brain metastases [3, 4]. However, small metastases that are invisible on treatment planning imaging are accordingly not irradiated. Combinations of SRS and TT or ICI have been used to overcome this limitation as they may also affect neoplastic cells outside irradiated volumes.
Sole SRS was shown to affect distant metastases also without concomitant immunotherapy. Even when its mechanisms are not fully understood, it is based on the release of cytokines and tumor cell antigens within irradiated metastases that stimulate a cytotoxic immune response [13]. It not only affects remaining tumor cells locally, but also acts on distant metastases [14]. This phenomenon is known as the ‘abscopal effect’ [15]. Investigations in mice and humans suggested that in CNS neoplasms, the abscopal effect be potentiated by ICI based on amplification of cellular immune mechanisms [16, 17].
Studies in patients with MM comparing radiosurgery and ICI or TT to radiosurgery alone showed that combined therapies have the potential to increase response and local control rates compared to sole radiosurgery [18,19,20,21,22]. However, interpretation of imaging findings in patients undergoing combined therapy may be challenging as treatment with both SRS and ICI or TT is known to have the potential to generate findings that may confuse with progressive disease, so called pseudoprogression. Pseudoprogression generally is indistinguishable from true progression in a single MRI study. It is diagnosed by serial imaging when findings remain stable or resolve without changing the therapy regime.
It is known that MM may possibly show a delayed response after immunotherapy, especially after administration of ipilimumab, thus immune-related Response Criteria (irRC) were introduced [23, 24]. These require the initial increase of at least 25% in lesion load to be confirmed by follow-up imaging at least 4 weeks later to diagnose progressive disease and exclude pseudoprogression. We therefore analyzed time dependent changes and MRI characteristics of treated lesions over a long time period up to 24 months.
In our study, in patients treated with ICI and ICI + TT, an early progression of MM volumes indicative of pseudoprogression was seen 2–4 months after SRS.
Even though in several patients medical therapy was initiated more than 6 weeks preceding SRS, the finding is plausible as delayed effects of immunotherapy with pseudoprogression up to 6 months after initiation have been described [25]. In contrast, pseudoprogression was also reported in patients in whom drug therapy was initiated within 6 weeks after SRS [26].
In our investigation, the finding of increased MM volumes after SRS however, was absent in patients that received TT simultaneously to SRS. This group showed a significant reduction in overall MM volumes starting at 2 months follow-up.
Patel et al. [27] investigated size changes in brain metastases of different primary tumors after sole radiosurgery and observed an increase in metastasis volumes in about one third of cases during the follow-up period beginning at 6 weeks post radiation ranging up to 15 months. Lesions’ volumes showed transient increases of 3.6% after 12 months and 11.6% after 15 months, respectively. In contrast, a recent study reported that 20% of MM post SRS had a temporary, reversible increase in size much earlier at 3–6 months after concomitant treatment with anti-PD-1 ICI, compared to 5% with radiosurgery alone [28].
Radiation necrosis is another important side effect of radiation therapy that is associated with an increase in lesional volume. Compared to pseudoprogression, radiation necrosis usually occurs later with a maximum of these changes reported to be reached after 12–18 months [27].
We observed a second increase in lesional volumes and perifocal edema volumes that peaked at 12–18 months after SRS in patients that received TT and ICI and TT. However, there was no clear association of this phenomenon with the occurrence or absence of early volume increase after SRS.
Corticosteroids have long been the mainstay for the treatment of radiation necrosis in brain metastases. They decrease inflammatory signals and cytokines produced by the necrotic tissue thereby reducing the leakiness of the blood–brain barrier, leading to a swift decrease in perilesional edema and contrast enhancement [29].
Bevacizumab, an anti-vascular endothelial growth factor (VEGF) monoclonal antibody that inhibits the pro-inflammatory response, is increasingly used. Bevacizumab may elicit a dramatic response and significant MRI findings with rapid volume reduction on both contrast enhanced T1-weighted and T2-weighted MR images and reported mean reduction rates in follow-up of 62.017 and 48.58% respectively [30]. According to a recent meta-analysis, bevacizumab can be considered safe and efficacious for the treatment of radiation necrosis in brain metastases, however, the level of evidence was low [30].
In our study total edema volumes mirrored time course of MM load with a maximum reached after 2–4 months, a finding which is in accordance with former reports. Jardim et al. [31] investigated post-SRS edema in patients with MM and found a transient increase in mean edema volume at 3 months after RS that resolved by 6 months. It did neither correlate with adverse events nor the need for steroids. Another study investigated edema volumes after SRS in patients who concomitantly received ipilimumab and found edema quantities that mirrored MM volumes at 1.5, 3, and 6-month following SRS [9]. Interestingly, in patients concomitantly treated with ICI, there was no more measurable edema at 6 months follow-up and thereafter.
In a study that investigated perilesional edema, treatment with ipilimumab additionally to SRS was shown to improve tumor and edema volumes, whereas it was associated with a higher incidence of metastatic bleeds [32].
We found newly emerging metastatic bleeds in first follow-up 2 months after SRS that occurred in 37/169 irradiated MMs (= 21.9%) in 8 patients. MM hemorrhages with longer intervals (> 3 months) to SRS were limited to 4 MMs. Investigations that studied the rates of bleeding in MM post SRS reported of pretreatment hemorrhage rates of 21.8–23.7% and post treatment hemorrhage rates of 15.2–20.7% [33, 34]. Rate of pretreatment MM hemorrhage in our study was 5.9%. This low rate may result from early detection of MM in our patient collective, as patients had routine follow-up brain MRI scans after initial diagnosis of melanoma.
Other investigators studied bleeding rates in cerebral non-small cell lung cancer metastases combined treatment with ICI and found no increased rate of intratumoral hemorrhage in patients receiving concurrent ICI after SRS compared to patients with SRS alone [35] whereas in patients with MM, simultaneously administration of ipilimumab was reported with a higher incidence of lesion hemorrhage [32].
Differentiation between progression and therapy-induced changes such as pseudoprogression and radiation necrosis in cerebral metastases may be challenging on conventional MRI due to the lack of unequivocal distinguishing features. In addition, advances MR methods such as MR perfusion or MR spectroscopy have also limited diagnostic accuracy to differentiate between therapy-associated brain changes and real tumor progression [36,37,38,39,40].
For a retrospective analysis, it is easier to differentiate between real tumor progression and therapy-induced changes by analyzing the time course of the lesion evolution and regression. Hence, we followed-up the lesions after the first progression and only in case of further progression seen in the follow-up MRI scans; the finding was defined as real progression.
However, this course was not observed in the irradiated MM in our study. In contrast, the initial progression was defined as therapy-induced when the size decreased in the course of the disease, whereby there is no sharp MR imaging criterion dividing radionecrosis and pseudoprogression.
Beside conventional MRI and advanced MR methods, PET may be helpful in distinguishing real progression from pseudoprogression [41,42,43,44,45], but in our collective, PWI, MRS or PET-imaging was not performed. The MRIs were acquired to plan and to navigate the possibly immediately following Cyberknife therapy. Further prospective and large-scaled studies should use a multimodal design to evaluate, if the combination of MR morphological feature, hemodynamic and metabolic information have a real diagnostic gain to distinguish between progression and therapy-induced changes.
Our study is limited by its retrospective layout and small heterogenous patient population. Patients were treated in a CyberKnife SRS center with a large catchment area, thus patients had different preceding therapy regimens and presented at different stages of the disease at the time of SRS treatment. Furthermore, there were no available data from MM patients that were solely treated with SRS. A larger cohort could be helpful to uncover possible differences regarding PD-1 and CTLA-4 effects within this setting.
In conclusion, in early follow-up after SRS, findings suggestive of pseudoprogression were found in patients who concomitantly received ICI or ICI + TT. Our results may be explained by synergistic and potentiating cellular effects of ICI after SRS that have recently been shown in CNS neoplasms in experimental and clinical setup.
Even if the median survival time in patients with ICI was not reached in the follow-up period after SRS, there is a clear tendency for these patients for a longer survival, compared to the group of patients treated with TT.
Data availability
The datasets generated during the current study are available from the corresponding author on reasonable request.
References
Murrell J, Board R (2013) The use of systemic therapies for the treatment of brain metastases in metastatic melanoma: opportunities and unanswered questions. Cancer Treat Rev 39:833–838. https://doi.org/10.1016/j.ctrv.2013.06.004
Davies MA, Liu P, McIntyre S et al (2011) Prognostic factors for survival in melanoma patients with brain metastases. Cancer 117:1687–1696. https://doi.org/10.1002/cncr.25634
Soffietti R, Abacioglu U, Baumert B et al (2017) Diagnosis and treatment of brain metastases from solid tumors: guidelines from the European Association of Neuro-Oncology (EANO). Neuro-Oncol 19:162–174. https://doi.org/10.1093/neuonc/now241
Tsao MN, Rades D, Wirth A et al (2012) Radiotherapeutic and surgical management for newly diagnosed brain metastasis(es): an American Society for Radiation Oncology evidence-based guideline. Pract Radiat Oncol 2:210–225. https://doi.org/10.1016/j.prro.2011.12.004
Tsao MN, Xu W, Wong RK et al (2018) Whole brain radiotherapy for the treatment of newly diagnosed multiple brain metastases. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD003869.pub4
Schvartsman G, Taranto P, Glitza IC et al (2019) Management of metastatic cutaneous melanoma: updates in clinical practice. Ther Adv Med Oncol 11:175883591985166. https://doi.org/10.1177/1758835919851663
Stera S, Balermpas P, Blanck O et al (2019) Stereotactic radiosurgery combined with immune checkpoint inhibitors or kinase inhibitors for patients with multiple brain metastases of malignant melanoma. Melanoma Res 29:187–195. https://doi.org/10.1097/CMR.0000000000000542
Stokes WA, Binder DC, Jones BL et al (2017) Impact of immunotherapy among patients with melanoma brain metastases managed with radiotherapy. J Neuroimmunol 313:118–122. https://doi.org/10.1016/j.jneuroim.2017.10.006
Diao K, Bian SX, Routman DM et al (2018) Stereotactic radiosurgery and ipilimumab for patients with melanoma brain metastases: clinical outcomes and toxicity. J Neurooncol 139:421–429. https://doi.org/10.1007/s11060-018-2880-y
Gabani P, Fischer-Valuck BW, Johanns TM et al (2018) Stereotactic radiosurgery and immunotherapy in melanoma brain metastases: patterns of care and treatment outcomes. Radiother Oncol J Eur Soc Ther Radiol Oncol 128:266–273. https://doi.org/10.1016/j.radonc.2018.06.017
Da Silva AN, Nagayama K, Schlesinger D, Sheehan JP (2009) Early brain tumor metastasis reduction following Gamma Knife surgery. J Neurosurg 110:547–552. https://doi.org/10.3171/2008.4.17537
Huber PE, Hawighorst H, Fuss M et al (2001) Transient enlargement of contrast uptake on MRI after linear accelerator (linac) stereotactic radiosurgery for brain metastases. Int J Radiat Oncol Biol Phys 49:1339–1349. https://doi.org/10.1016/s0360-3016(00)01511-x
Yilmaz MT, Elmali A, Yazici G (2019) Abscopal effect, from myth to reality: from radiation oncologists’ perspective. Cureus. https://doi.org/10.7759/cureus.3860
Quail DF, Joyce JA (2017) The microenvironmental landscape of brain tumors. Cancer Cell 31:326–341. https://doi.org/10.1016/j.ccell.2017.02.009
Pfannenstiel LW, McNeilly C, Xiang C et al (2019) Combination PD-1 blockade and irradiation of brain metastasis induces an effective abscopal effect in melanoma. OncoImmunology 8:e1507669. https://doi.org/10.1080/2162402X.2018.1507669
D’Souza NM, Fang P, Logan J et al (2016) Combining radiation therapy with immune checkpoint blockade for central nervous system malignancies. Front Oncol. https://doi.org/10.3389/fonc.2016.00212
Ene CI, Kreuser SA, Jung M et al (2019) Anti–PD-L1 antibody direct activation of macrophages contributes to a radiation-induced abscopal response in glioblastoma. Neuro-Oncol. https://doi.org/10.1093/neuonc/noz226
Acharya S, Mahmood M, Mullen D et al (2017) Distant intracranial failure in melanoma brain metastases treated with stereotactic radiosurgery in the era of immunotherapy and targeted agents. Adv Radiat Oncol 2:572–580. https://doi.org/10.1016/j.adro.2017.07.003
Ahmed KA, Abuodeh YA, Echevarria MI et al (2016) Clinical outcomes of melanoma brain metastases treated with stereotactic radiosurgery and anti-PD-1 therapy, anti-CTLA-4 therapy, BRAF/MEK inhibitors, BRAF inhibitor, or conventional chemotherapy. Ann Oncol 27:2288–2294. https://doi.org/10.1093/annonc/mdw417
Kotecha R, Miller JA, Venur VA et al (2018) Melanoma brain metastasis: the impact of stereotactic radiosurgery, BRAF mutational status, and targeted and/or immune-based therapies on treatment outcome. J Neurosurg 129:50–59. https://doi.org/10.3171/2017.1.JNS162797
Patel KR, Shoukat S, Oliver DE et al (2017) Ipilimumab and stereotactic radiosurgery versus stereotactic radiosurgery alone for newly diagnosed melanoma brain metastases. Am J Clin Oncol 40:444–450. https://doi.org/10.1097/COC.0000000000000199
Wilhelm M-L, Chan MKH, Abel B et al (2020) Tumor-dose-rate variations during robotic radiosurgery of oligo and multiple brain metastases. Strahlenther Onkol Organ Dtsch Rontgengesellschaft Al. https://doi.org/10.1007/s00066-020-01652-6
Wolchok JD, Hoos A, O’Day S et al (2009) Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res 15:7412–7420. https://doi.org/10.1158/1078-0432.CCR-09-1624
Dromain C, Beigelman C, Pozzessere C et al (2020) Imaging of tumour response to immunotherapy. Eur Radiol Exp 4:2. https://doi.org/10.1186/s41747-019-0134-1
Galldiks N, Kocher M, Ceccon G et al (2019) Imaging challenges of immunotherapy and targeted therapy in patients with brain metastases: response, progression, and pseudoprogression. Neuro-Oncol. https://doi.org/10.1093/neuonc/noz147
Nordmann N, Hubbard M, Nordmann T et al (2017) Effect of gamma knife radiosurgery and programmed cell death 1 receptor antagonists on metastatic melanoma. Cureus 9:e1943. https://doi.org/10.7759/cureus.1943
Patel TR, McHugh BJ, Bi WL et al (2011) A comprehensive review of MR imaging changes following radiosurgery to 500 brain metastases. Am J Neuroradiol 32:1885–1892. https://doi.org/10.3174/ajnr.A2668
Trommer-Nestler M, Marnitz S, Kocher M et al (2018) Robotic stereotactic radiosurgery in melanoma patients with brain metastases under simultaneous anti-PD-1 treatment. Int J Mol Sci 19:2653. https://doi.org/10.3390/ijms19092653
Vellayappan B, Tan CL, Yong C et al (2018) Diagnosis and management of radiation necrosis in patients with brain metastases. Front Oncol 8:395. https://doi.org/10.3389/fonc.2018.00395
Khan M, Zhao Z, Arooj S, Liao G (2021) Bevacizumab for radiation necrosis following radiotherapy of brain metastatic disease: a systematic review & meta-analysis. BMC Cancer 21:167. https://doi.org/10.1186/s12885-021-07889-3
Jardim A, Scott J, Drew Z et al (2019) Extent of surrounding edema does not correlate with acute complications after radiosurgery for melanoma brain metastases. J Neurooncol 145:581–585. https://doi.org/10.1007/s11060-019-03330-9
Diao K, Bian SX, Routman DM et al (2018) Combination ipilimumab and radiosurgery for brain metastases: tumor, edema, and adverse radiation effects. J Neurosurg 129:1397–1406. https://doi.org/10.3171/2017.7.JNS171286
Redmond AJ, DiLuna ML, Hebert R et al (2008) Gamma Knife surgery for the treatment of melanoma metastases: the effect of intratumoral hemorrhage on survival. J Neurosurg 109:99–105. https://doi.org/10.3171/JNS/2008/109/12/S16
Ghia AJ, Tward JD, Anker CJ et al (2014) Radiosurgery for melanoma brain metastases: the impact of hemorrhage on local control. J Radiosurgery SBRT 3:43–50
Shepard MJ, Xu Z, Donahue J et al (2019) Stereotactic radiosurgery with and without checkpoint inhibition for patients with metastatic non–small cell lung cancer to the brain: a matched cohort study. J Neurosurg. https://doi.org/10.3171/2019.4.JNS19822
Chuang M-T, Liu Y-S, Tsai Y-S et al (2016) Differentiating radiation-induced necrosis from recurrent brain tumor using MR perfusion and spectroscopy: a meta-analysis. PLoS ONE 11:e0141438. https://doi.org/10.1371/journal.pone.0141438
Huang J, Wang A-M, Shetty A et al (2011) Differentiation between intra-axial metastatic tumor progression and radiation injury following fractionated radiation therapy or stereotactic radiosurgery using MR spectroscopy, perfusion MR imaging or volume progression modeling. Magn Reson Imaging 29:993–1001. https://doi.org/10.1016/j.mri.2011.04.004
Chernov MF, Hayashi M, Izawa M et al (2006) Multivoxel proton MRS for differentiation of radiation-induced necrosis and tumor recurrence after gamma knife radiosurgery for brain metastases. Brain Tumor Pathol 23:19–27. https://doi.org/10.1007/s10014-006-0194-9
Hellström J, Romanos Zapata R, Libard S et al (2018) The value of magnetic resonance spectroscopy as a supplement to MRI of the brain in a clinical setting. PLoS ONE 13:e0207336. https://doi.org/10.1371/journal.pone.0207336
Kerkhof M, Ganeff I, Wiggenraad RGJ et al (2018) Clinical applicability of and changes in perfusion MR imaging in brain metastases after stereotactic radiotherapy. J Neurooncol 138:133–139. https://doi.org/10.1007/s11060-018-2779-7
Terakawa Y, Tsuyuguchi N, Iwai Y et al (2008) Diagnostic accuracy of 11C-methionine PET for differentiation of recurrent brain tumors from radiation necrosis after radiotherapy. J Nucl Med 49:694–699. https://doi.org/10.2967/jnumed.107.048082
Lizarraga KJ, Allen-Auerbach M, Czernin J et al (2014) (18)F-FDOPA PET for differentiating recurrent or progressive brain metastatic tumors from late or delayed radiation injury after radiation treatment. J Nucl Med 55:30–36. https://doi.org/10.2967/jnumed.113.121418
Cicone F, Minniti G, Romano A et al (2015) Accuracy of F-DOPA PET and perfusion-MRI for differentiating radionecrotic from progressive brain metastases after radiosurgery. Eur J Nucl Med Mol Imaging 42:103–111. https://doi.org/10.1007/s00259-014-2886-4
Galldiks N, Stoffels G, Filss CP et al (2012) Role of O-(2-(18)F-fluoroethyl)-L-tyrosine PET for differentiation of local recurrent brain metastasis from radiation necrosis. J Nucl Med 53:1367–1374. https://doi.org/10.2967/jnumed.112.103325
Ceccon G, Lohmann P, Stoffels G et al (2017) Dynamic O-(2–18F-fluoroethyl)-L-tyrosine positron emission tomography differentiates brain metastasis recurrence from radiation injury after radiotherapy. Neuro-Oncol 19:281–288. https://doi.org/10.1093/neuonc/now149
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
MR: Conceptualization; Methodology; Software; Validation; Formal analysis; Investigation; Resources; Data Curation; Writing—Original Draft; Visualization; Project administration; DT: Software; Validation; Formal analysis; Investigation; Data Curation; SS: Validation; Investigation; Resources; Data Curation; Writing—Review & Editing; OB: Validation; Investigation; Resources; Data Curation; Writing—Review & Editing; RW: Validation; Investigation; Resources; Data Curation; Writing—Review & Editing; MM: Validation; Investigation; Resources; Writing—Review & Editing; Hans Urban: Validation; Investigation; Resources; Writing—Review & Editing; EH: Conceptualization; Methodology; Validation; Formal analysis; Investigation; Resources; Data Curation; Writing—Review & Editing; Supervision; Project administration.
Corresponding author
Ethics declarations
Conflict of interest
Related to the present work, the authors have no potential conflicts of interest to declare.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The retrospective study was approved by the ethics committee of the Johann Wolfgang Goethe-University Frankfurt (Number 395/17).
Consent to participate
Consent to participate was waived due to the retrospective study design.
Consent for publication
Consent for publication was waived due to the retrospective study design.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rauch, M., Tausch, D., Stera, S. et al. MRI characteristics in treatment for cerebral melanoma metastasis using stereotactic radiosurgery and concomitant checkpoint inhibitors or targeted therapeutics. J Neurooncol 153, 79–87 (2021). https://doi.org/10.1007/s11060-021-03744-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-021-03744-4