Abstract
Introduction
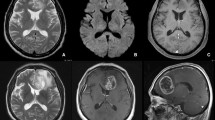
Magnet resonance imaging (MRI) of gliomas is assessed by Response Assessment in Neuro-Oncology Criteria (RANO), which define new contrast-enhancing lesions as a sign for tumor recurrence. Pseudoprogression after radiotherapy may mimic tumor progression in MRI but is usually limited to the first months after irradiation. We noted a late onset pattern of new contrast-enhancing spots (NCES) appearing years after radiotherapy.
Methods
We prospectively collected 23 glioma patients with 26 NCES (three patients had two separate NCES events) between 2014 and 2016 in our weekly tumor board without further selection by diagnosis, molecular markers or pretreatment.
Results
Retrospective analysis revealed a homogeneous collective of young patients (median age of 49 years at NCES) with mainly IDH-mutated glioma (61%). Initial histology showed 26% glioblastoma, 52% grade III and 22% grade II glioma. NCES occurred at late follow-up with a median of 52 months after tumor diagnosis and 30 months after the last radiotherapy. The majority of NCES regressed spontaneously within a median of 10 months (n = 11) or remained stable without further therapy with a median follow-up of 26 months (n = 7). Only 4 NCES developed MRI morphologically into tumor recurrence. Two NCES were resected without any histopathological proof of tumor recurrence, and in 2 other cases NCES were defined as ischemic stroke or radionecrosis.
Conclusion
We hypothesize that the late onset phenomenon of NCES predominantly represents a form of radiation-induced vasculopathy that is different from early pseudoprogression and should be considered especially in younger patients with IDH-mutated glioma before initiation of new therapy.




Similar content being viewed by others
References
Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD, Kleihues P, Ellison DW (2016) The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol 131:803–820. https://doi.org/10.1007/s00401-016-1545-1
Wen PY, Macdonald DR, Reardon DA, Cloughesy TF, Sorensen AG, Galanis E, Degroot J, Wick W, Gilbert MR, Lassman AB, Tsien C, Mikkelsen T, Wong ET, Chamberlain MC, Stupp R, Lamborn KR, Vogelbaum MA, van den Bent MJ, Chang SM (2010) Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol 28:1963–1972. https://doi.org/10.1200/JCO.2009.26.3541
Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO, European Organisation for R, Treatment of Cancer Brain T, Radiotherapy G, National Cancer Institute of Canada Clinical Trials G (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352: 987–996 https://doi.org/10.1056/NEJMoa043330
Brandes AA, Franceschi E, Tosoni A, Blatt V, Pession A, Tallini G, Bertorelle R, Bartolini S, Calbucci F, Andreoli A, Frezza G, Leonardi M, Spagnolli F, Ermani M (2008) MGMT promoter methylation status can predict the incidence and outcome of pseudoprogression after concomitant radiochemotherapy in newly diagnosed glioblastoma patients. J Clin Oncol 26:2192–2197. https://doi.org/10.1200/JCO.2007.14.8163
Gahramanov S, Varallyay C, Tyson RM, Lacy C, Fu R, Netto JP, Nasseri M, White T, Woltjer RL, Gultekin SH, Neuwelt EA (2014) Diagnosis of pseudoprogression using MRI perfusion in patients with glioblastoma multiforme may predict improved survival. CNS Oncol 3:389–400. https://doi.org/10.2217/cns.14.42
Ranjan S, Quezado M, Garren N, Boris L, Siegel C, Lopes Abath Neto O, Theeler BJ, Park DM, Nduom E, Zaghloul KA, Gilbert MR, Wu J (2018) Clinical decision making in the era of immunotherapy for high grade-glioma: report of four cases. BMC Cancer 18:239. https://doi.org/10.1186/s12885-018-4131-1
Siu A, Wind JJ, Iorgulescu JB, Chan TA, Yamada Y, Sherman JH (2012) Radiation necrosis following treatment of high grade glioma–a review of the literature and current understanding. Acta Neurochir (Wien) 154:191–201. https://doi.org/10.1007/s00701-011-1228-6 (Discussion 201)
Babu R, Huang PP, Epstein F, Budzilovich GN (1993) Late radiation necrosis of the brain: case report. J Neurooncol 17:37–42
Blasel S, Franz K, Mittelbronn M, Morawe G, Jurcoane A, Pellikan S, Zanella F, Hattingen E (2010) The striate sign: peritumoural perfusion pattern of infiltrative primary and recurrent gliomas. Neurosurg Rev 33:193–203. https://doi.org/10.1007/s10143-010-0248-7 (Discussion 203–194)
Boxerman JL, Schmainda KM, Weisskoff RM (2006) Relative cerebral blood volume maps corrected for contrast agent extravasation significantly correlate with glioma tumor grade, whereas uncorrected maps do not. AJNR Am J Neuroradiol 27:859–867
Kumar AJ, Leeds NE, Fuller GN, Van Tassel P, Maor MH, Sawaya RE, Levin VA (2000) Malignant gliomas: MR imaging spectrum of radiation therapy- and chemotherapy-induced necrosis of the brain after treatment. Radiology 217:377–384. https://doi.org/10.1148/radiology.217.2.r00nv36377
Li YQ, Chen P, Haimovitz-Friedman A, Reilly RM, Wong CS (2003) Endothelial apoptosis initiates acute blood-brain barrier disruption after ionizing radiation. Cancer Res 63:5950–5956
van den Ameele J, Sieben A, Van den Broecke C, Boterberg T, Defreyne L, Achten E, Lammens M, Hemelsoet D (2012) Late-onset post-irradiation vasculopathy of the posterior cerebral vasculature. Acta Neurol Belg 112:101–104. https://doi.org/10.1007/s13760-012-0014-4
Miura M, Nakajima M, Fujimoto A, Kaku Y, Kawano T, Watanabe M, Kuratsu JI, Ando Y (2017) High prevalence of small vessel disease long after cranial irradiation. J Clin Neurosci 46:129–135. https://doi.org/10.1016/j.jocn.2017.09.008
Yoshii Y (2008) Pathological review of late cerebral radionecrosis. Brain Tumor Pathol 25:51–58. https://doi.org/10.1007/s10014-008-0233-9
Gaensler EH, Dillon WP, Edwards MS, Larson DA, Rosenau W, Wilson CB (1994) Radiation-induced telangiectasia in the brain simulates cryptic vascular malformations at MR imaging. Radiology 193:629–636. https://doi.org/10.1148/radiology.193.3.7972799
Pozzati E, Giangaspero F, Marliani F, Acciarri N (1996) Occult cerebrovascular malformations after irradiation. Neurosurgery 39:677–682 (Discussion 682–674)
Miyatake S, Nonoguchi N, Furuse M, Yoritsune E, Miyata T, Kawabata S, Kuroiwa T (2015) Pathophysiology, Diagnosis, and Treatment of Radiation Necrosis in the Brain. Neurol Med Chir (Tokyo) 55(Suppl 1):50–59
Levin VA, Bidaut L, Hou P, Kumar AJ, Wefel JS, Bekele BN, Grewal J, Prabhu S, Loghin M, Gilbert MR, Jackson EF (2011) Randomized double-blind placebo-controlled trial of bevacizumab therapy for radiation necrosis of the central nervous system. Int J Radiat Oncol Biol Phys 79:1487–1495. https://doi.org/10.1016/j.ijrobp.2009.12.061
Chen H, Judkins J, Thomas C, Wu M, Khoury L, Benjamin CG, Pacione D, Golfinos JG, Kumthekar P, Ghamsari F, Chen L, Lein P, Chetkovich DM, Snuderl M, Horbinski C (2017) Mutant IDH1 and seizures in patients with glioma. Neurology 88:1805–1813. https://doi.org/10.1212/WNL.0000000000003911
Xu W, Yang H, Liu Y, Yang Y, Wang P, Kim SH, Ito S, Yang C, Wang P, Xiao MT, Liu LX, Jiang WQ, Liu J, Zhang JY, Wang B, Frye S, Zhang Y, Xu YH, Lei QY, Guan KL, Zhao SM, Xiong Y (2011) Oncometabolite 2-hydroxyglutarate is a competitive inhibitor of alpha-ketoglutarate-dependent dioxygenases. Cancer Cell 19:17–30. https://doi.org/10.1016/j.ccr.2010.12.014
Zhao S, Lin Y, Xu W, Jiang W, Zha Z, Wang P, Yu W, Li Z, Gong L, Peng Y, Ding J, Lei Q, Guan KL, Xiong Y (2009) Glioma-derived mutations in IDH1 dominantly inhibit IDH1 catalytic activity and induce HIF-1alpha. Science 324:261–265. https://doi.org/10.1126/science.1170944
Li H, Li J, Cheng G, Zhang J, Li X (2016) IDH mutation and MGMT promoter methylation are associated with the pseudoprogression and improved prognosis of glioblastoma multiforme patients who have undergone concurrent and adjuvant temozolomide-based chemoradiotherapy. Clin Neurol Neurosurg 151:31–36. https://doi.org/10.1016/j.clineuro.2016.10.004
Lin AL, White M, Miller-Thomas MM, Fulton RS, Tsien CI, Rich KM, Schmidt RE, Tran DD, Dahiya S (2016) Molecular and histologic characteristics of pseudoprogression in diffuse gliomas. J Neurooncol 130:529–533. https://doi.org/10.1007/s11060-016-2247-1
Juratli TA, Engellandt K, Lautenschlaeger T, Geiger KD, von Kummer R, Cerhova J, Chakravarti A, Krex D, Schackert G (2013) Is there pseudoprogression in secondary glioblastomas? Int J Radiat Oncol Biol Phys 87:1094–1099. https://doi.org/10.1016/j.ijrobp.2013.09.036
Lin AL, Liu J, Evans J, Leuthardt EC, Rich KM, Dacey RG, Dowling JL, Kim AH, Zipfel GJ, Grubb RL, Huang J, Robinson CG, Simpson JR, Linette GP, Chicoine MR, Tran DD (2014) Codeletions at 1p and 19q predict a lower risk of pseudoprogression in oligodendrogliomas and mixed oligoastrocytomas. Neuro Oncol 16:123–130. https://doi.org/10.1093/neuonc/not142
Hu LS, Baxter LC, Smith KA, Feuerstein BG, Karis JP, Eschbacher JM, Coons SW, Nakaji P, Yeh RF, Debbins J, Heiserman JE (2009) Relative cerebral blood volume values to differentiate high-grade glioma recurrence from posttreatment radiation effect: direct correlation between image-guided tissue histopathology and localized dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging measurements. AJNR Am J Neuroradiol 30:552–558. https://doi.org/10.3174/ajnr.A1377
Kong DS, Kim ST, Kim EH, Lim DH, Kim WS, Suh YL, Lee JI, Park K, Kim JH, Nam DH (2011) Diagnostic dilemma of pseudoprogression in the treatment of newly diagnosed glioblastomas: the role of assessing relative cerebral blood flow volume and oxygen-6-methylguanine-DNA methyltransferase promoter methylation status. AJNR Am J Neuroradiol 32:382–387. https://doi.org/10.3174/ajnr.A2286
Blasel S, Zagorcic A, Jurcoane A, Bahr O, Wagner M, Harter PN, Hattingen E (2016) Perfusion MRI in the Evaluation of Suspected Glioblastoma Recurrence. J Neuroimaging 26:116–123. https://doi.org/10.1111/jon.12247
Bian W, Hess CP, Chang SM, Nelson SJ, Lupo JM (2014) Susceptibility-weighted MR imaging of radiation therapy-induced cerebral microbleeds in patients with glioma: a comparison between 3T and 7T. Neuroradiology 56:91–96. https://doi.org/10.1007/s00234-013-1297-8
Lupo JM, Chuang CF, Chang SM, Barani IJ, Jimenez B, Hess CP, Nelson SJ (2012) 7-Tesla susceptibility-weighted imaging to assess the effects of radiotherapy on normal-appearing brain in patients with glioma. Int J Radiat Oncol Biol Phys 82:e493–e500. https://doi.org/10.1016/j.ijrobp.2011.05.046
Acknowledgements
The Senckenberg Institute of Neurooncology is supported by the Senckenberg Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
This study has been accepted by the local ethics committee (SNO_SNO_01–08).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Voss, M., Franz, K., Steinbach, J.P. et al. Contrast enhancing spots as a new pattern of late onset pseudoprogression in glioma patients. J Neurooncol 142, 161–169 (2019). https://doi.org/10.1007/s11060-018-03076-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-018-03076-w