Abstract
Treatment of infant hypothalamic chiasmatic glioma (iCHG) is challenging, about 30% of the children progress during chemotherapy. Despite subsequent treatments the 5 year overall-survival rate is only 70%. This study investigates treatment strategies currently applied for progressive iCHG. A web-based questionnaire was sent out to the members of the SIOPE Brain Tumour Group asking for current second and third line strategies at progression during and after the end of first line therapy. The questionnaire was answered by 47 paediatric oncologists from 15 countries. iCHG progressing during first line therapy with carboplatin-vincristine would be considered for treatment with alternative chemotherapy by 17 (36%) and with surgery plus chemotherapy by 27 respondents (58%). Components suggested for second line were vinblastine (62%), cisplatin (34%) and cyclophosphamide (26%). For third line therapy bevacizumab (BVZ) was considered as suitable by respondents in 53% (often with irinotecan 40%) and vinblastine by 34% respectively. Experience with BVZ in CHG is shown by 53% of respondents regarding at least 95 patients (median treated 1–5 patients per respondent at any age) with a median BVZ administration over 12 months. Effectiveness was reported varying between stable disease and regression while complications were rarely stated (proteinuria, hypertension, bleeding). BVZ would be available to 85% of respondents as therapeutic option for iCHG patients. Multiple anti-neoplastic drug regimens are applied for progressive iCHG, partly considered in combination with surgery if safely feasible. BVZ is commonly used at a satisfactory level in third line, mainly combined with irinotecan.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Low-grade gliomas (LGG) are the most common brain tumours in childhood, accounting for approximately 35% during the first year of life and up to 50% in older children [1]. The chiasmatic/hypothalamic region is the predilective location in infants, and 20% of all intracranial tumours in children below the age of 2 years occur in this region [2]. Overall, infant chiasmatic hypothalamic glioma (iCHG) still constitutes a rare entity [3]. Young age has been demonstrated to represent a risk factor for progression in children with LGG, translating into an inferior overall survival rate [4,5,6]. Whereas surgical resection improves outcome in LGG outside of the chiasmatic/hypothalamic region [4, 5], it does not in children with iCHG and may cause additional harm such as infarction or impaired neurological and endocrine function [7, 8]. The vulnerability of the very young brain hampers the application of radiotherapy at this age [9,10,11]. Systemic chemotherapy is required and the regimen most commonly used in first-line comprises carboplatin and vincristine in different time-schedules and dosing [5, 12]. Treatment with carboplatin and vincristine over a period of 18 months represents the current strategy proposed by the Societé Internationale d’Oncologie Pédiatrique in Europe (SIOPE) [13]. iCHG is characterised by multiple sequential progressions and the necessity to apply subsequent treatment cycles [3, 6, 14, 15]. Multiple different therapy regimens have been reported for progressive LGG, including monotherapies with vinblastine [16], temozolomide [17], or carboplatin [18, 19] as well as chemotherapy combinations such as TPCV (thioguanine, procarbazine, CCNU (1-(2-chloroethyl)-3-cyclohexyl-1-nitrosourea) and vincristine) [15] or repeated carboplatin/vincristine [15]. These relapse strategies were shown to efficiently influence survival.
The present survey was conducted following intense discussions among the SIOPE LGG working group on possible treatment strategies for infants with progressive CHG. The survey therefore aimed at evaluating treatment strategies currently adopted for progressive iCHG, i.e. progressive tumour in children diagnosed during the first year of life. Following primary reports on the efficacy of bevacizumab (BVZ) in patients with LGG [20,21,22,23], and the inefficacy of conventional chemotherapy in approximately 30% of iCHG patients, the use of BVZ was discussed as possible treatment strategy. The working group recognised the lack of data for the use of BVZ in such a vulnerable young patient cohort, necessitating a detailed evaluation of its use in iCHG patients. The current professional experiences in application of BVZ in paediatric CHG were therefore investigated in more detail.
Methods
A web-based questionnaire on experiences and preferences on relapse treatment for iCHG (during or following first line treatment with carboplatin/vincristine) was created at http://www.thesistools.de (see supplementary material). Per definition, tumours were considered as infant CHG if diagnosed during the first year of life. Treatment at progression or at relapse after the end of intial therapy (independent of the age at relapse) was addressed in the present survey. The survey took around 15 min to fill in and consisted of questions regarding treatment strategies, drugs applied for systemic therapy and experience with bevacizumab in CHG patients. Additional questions were asked on characteristics of the participating medical specialist: profession, country of origin, years of clinical experience, yearly amount of children treated with a brain tumour and the subgroups of LGG, CHG as well as iCHG specifically. As the survey aimed at assessing general treatment strategies, it did not collect data on patient characteristics (e.g. age at relapse, pattern of relapse or initial symptoms such as diencephalic syndrome).
The invitation to participate in the survey with a directly accessible link was sent to specialists in the field of paediatric neuro-oncology consisting of the 220 members of the multidisciplinary SIOPE Brain Tumour Group via email in February 2014 and repeated after 3 weeks.
Results
Respondents
The questionnaire was answered by 47 paediatric oncologists from 15 countries (Austria, Canada, Czech Republic, Denmark, France, Germany, Italy, Netherlands, Poland, Portugal, Slovakia, Spain, Sweden, United Kingdom, United States). Two neuro-surgeons were omitted from the analysis as they indicated not to be involved in the decision on systemic treatment approaches. The professional experience of the respondents was reflected by the fact that 85% worked in a clinic with > 20 new brain tumour patients per year. Their years of experience in neuro-oncology were as follows: <5 years n = 5, 5–10 years n = 8, 10–20 years n = 18, > 20 years n = 15 and missing n = 1. 81% treated 1–6 patients with newly diagnosed CHG yearly, 19% seven or more patients per year. The reported rate of iCHG was low, during a 5 year period 57% of respondents cared for 1–3, 23% for 4–6, while 20% for more than six patients with iCHG.
Duration of first line treatment
Considering the high rate of patients progressing after chemotherapy, the survey asked on the opinion of prolonging first line chemotherapy (see Table 1). The majority of respondents (81%) administered first line treatment over a period of 18 months, a third (36%) indicated to be willing to consider prolonging first line treatment. Vinblastine was the most prevalent suggestion for such a prolongation.
Treatment strategies at progression
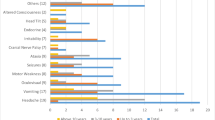
Therapeutic strategies adopted and systemic therapies administered at progression in patients with iCHG are summarised in Table 1. Progressive disease during first line therapy with carboplatin - vincristine would be treated with different chemotherapy by 17 (36%) and with surgery plus chemotherapy by 27 respondents (58%). Vinblastine was selected as single chemotherapeutic agent in 62% followed by cisplatin (34%) and cyclophosphamide (26%) in multi-agent combinations.
In case of progression after the end of combined carboplatin—vincristine chemotherapy 54% of respondents would favour vinblastine monotherapy while about 44% would choose a platinum derivative, 35% vincristine and 20% cyclophosphamide, mostly in combination therapy. In addition to systemic therapy 38% of respondents would consider a neurosurgical option (if safely feasible) in combination with further chemotherapy.
In third line respondents would use BVZ in 53%, in 19/25 (76%) combined with irinotecan. Vinblastine is considered by 34%.
The actual experiences in application of BVZ are listed in Table 2. Bevacizumab was applied in at least 95 patients with CHG by 53% of respondents, mostly combined with irinotecan. Median duration of BVZ therapy was 12 months (range 4–24). An effect was reported for all patients with at least stable disease while severe complications were rarely mentioned (proteinuria, hypertension and bleeding, each indicated by a single respondent). Although BVZ is not licensed for use in LGG, 85% of respondents stated that BVZ would be available at their centre for use in patients with progressive iCHG.
Other findings
Radiotherapy was not considered an option in second and third line therapy (see Table 1). Respondents indicated that the median age at which they would consider starting external beam radiotherapy was 7 years (range 1–18, “never” n = 2). Reasons to start radiotherapy were collected as open answers and are therefore challenging to evaluate. Forty-one out of 45 evaluable respondents would only consider radiotherapy if the tumour was chemo-irresponsive (most indicating failure to more than two to three lines of systemic therapy).
Discussion
iCHG has a high risk of progression (already occurring during chemotherapy in 70% of patients) and exhibits a poor overall survival of 70% [3,4,5,6]. Due to the very low incidence of iCHG prospective studies specifically targeting the infant population are difficult to imply and data on treatment efficacy are scarce [3]. Consequently there are no clear guidelines for relapse therapy and so far no drug choice has shown superior effectiveness. Opinions from experts in the field are highly relevant to increase insight in current practice. As patients with iCHG suffer multiple relapses [14] the present survey was conducted among the SIOPE Brain Tumour Group to identify presently applied treatment strategies, specifically the choices made in application of chemotherapeutic drugs. By inviting all members of the SIOPE Brain Tumour Group the present survey was reaching the broad SIOPE community of paediatric neuro-oncologists, reflected by respondents from 15 countries.
According to the current recommendations of the SIOPE LGG group, carboplatin and vincristine are mostly administered over a period of 18 months in first line therapy, but a third of responding experts would consider prolongation of first line treatment to ameliorate PFS.
The present survey underlines that systemic therapies are considered the mainstay of relapse therapy in iCHG. Even if neurosurgery is (re-)considered, subsequent systemic therapy approaches are deemed necessary to sustain a possible tumour reduction achieved by neurosurgery. Accordingly none of the respondents would select a stand-alone neurosurgical approach at first tumour progression and 6% at second progression.
Radiotherapy was not considered an option in second and third line therapy by respondents of this survey. The vast majority would even only consider radiotherapy after failure of multiple lines of systemic therapy. This reasoning will probably be based on the possible devastating side effects of cranial radiotherapy in small children [10, 11]. Also radiotherapy has to be considered a major risk factor for delayed mortality in adult survivors of childhood low-grade glioma [9].
The survey revealed that a wide variety of anti-neoplastic agents are applied in subsequent therapy lines.
At first progression vinblastine is chosen most often as 2nd line treatment, 62% for progression during carboplatin-vincristine and 54% for progression after the end first line treatment. The excellent tolerability and efficacy of vinblastine in LGG has been documented earlier and its efficacy was also reported in young children with iCHG and those with diencephalic syndrome, either alone or in combination with carboplatin [16, 24, 25]. Vinblastine was recently even reported as maintenance after BVZ-irinotecan therapy [26].
A wide variety of drugs is used in case of progression during and/or after first line chemotherapy. We could not define specific subgroups of professionals for instance by nationality and/or years of experience, who provided specific alternative chemotherapy regimens. Only the application of cisplatin in combination with etoposide was reported by Italian neuro-oncologists as published by Massimino et al. [27, 28] Although the decreased dose diminished ototoxicity [28], it still seems potentially ototoxic which is especially relevant in children with impaired vision where dual sensory loss is risked. The use of more intense chemotherapy regimens might reflect the fact that children with iCHG progressing under chemotherapy exhibit an increased mortality. The data also mirror the advice in the earlier LGG 2004 protocol to start cyclophosphamide and cisplatin alternating in cycles in parallel to vincristine in case of carboplatin allergy. The choices for actinomycin and methotrexate are scarce, although relevance has been shown in older literature reports [29,30,31].
The antiangiogenic agent BVZ, a monoclonal antibody targeting vascular endothelial growth factor is approved for oncologic treatment in adults and was consecutively applied in a variety of paediatric CNS malignancies. In childhood LGG several studies demonstrated benefit of BVZ, specifically in patients with CHG. Packer et al. were the first to report clinical and radiologic responses in ten cases with multiple relapsed LGG with a combination of two-weekly BVZ and irinotecan [20]. Kalra et al. showed similar results in 16 children, including 8 with disseminated disease [32]. It was demonstrated that a majority of patients relapsed after stopping therapy, but re-introduction of BVZ was able to re-induce response and maintained responses were seen after prolonging intervals to 3 weeks [23]. Besides tumour control, improvement of visual function was reported in eight out of nine patients (four stable vision, four improved vision) with previous visual loss from both studies [22, 32]. The toxicity profile of BVZ in children seems acceptable with temporary proteinuria, hypertension, impaired wound healing and rarely bleeding or osteonecrosis [23, 33,34,35,36].
Indeed application of BVZ for progressive iCHG seems to be a hopeful strategy as shown in the experiences of 53% of our respondents reflecting experience in at least 95 patients. The previously published results of BVZ in LGG incited us to investigate the current practice of BVZ application in progressive CHG in the clinical practice of paediatric neuro-oncology. The results of the survey suggest that while vinblastine is widely applied in second line, BVZ was indicated as most prevalent choice in third line therapy with a majority of clinical responses despite multiple progressions of their LGG.
BVZ is not approved for the use in children with LGG and expensive compared to conventional chemotherapy. Considering its effectiveness in LGG [20, 21] and the possible functional benefit of visual improvement in children with iCHG [22, 23], its use may still be considered. Results from an ongoing randomised phase 2 trial on BVZ and vinblastine in chemotherapy-naïve children with LGG (NCT02840409) are eagerly awaited.
Following the increasing understanding of molecular pathways involved in LGG pathogenesis [37], a novel treatment strategy targeting at the MAPK pathway was evaluated. However, despite early hope, RAF inhibitor sorafenib revealed not only ineffective, but proved even detrimental due to an activating feed-back loop causing rapid progression of recurrent LGG [38]. MEK inhibitors block signalling of the MAPK pathway downstream of RAF, thereby thought to circumvent the problems encountered in first generation BRAF inhibitors. Primary clinical data have been presented on the efficacy of MEK inhibitors in relapsed LGG, and results from phase 1 and 2 trials in the paediatric population are emerging [39, 40].
Since a majority of patients with LGG become long term survivors, more insight is needed in the late outcome by gathering data in a common registry [41]. This is specifically true for assessing long term sequelae resulting in children with iCHG caused by the tumour itself or its subsequent treatment. We therefore advocate a registry for all children with multiple subsequent treatment strategies at progression of CHG.
Strengths and limitations
Our survey provides insights in the opinions of a broad group of experienced paediatric neuro-oncologists and reflects clinical practice from many different countries in the choices made in treatment of progressive iCHG. The information regarding BVZ in clinical practice refers to at least 95 patients, extending the information on the use of BVZ, so far limited to reports published on 25 CHG patients treated with BVZ. Due to the nature of an online survey there was the possibility that multiple respondents from one treatment centre may have answered the questionnaire. Indeed, two IP-addresses were used by two respondents each. All four could be identified as independent respondents; one of them was neurosurgeon and left out from analysis (see above). Only 47/220 persons invited via email have responded to the survey, maybe distorting the results. As the mailing list of SIOPE also included neurologists, radiotherapists, neurosurgeons, neuro-pathologists, psychologists, endocrinologists, statisticians, basic scientists and ophthalmologists who were not addressed by the present survey, the percentage of paediatric neuro-oncologists responding is much higher than suggested by this number.
The efficacy of other targeted drugs, such as specification of MEK inhibitors were not implicated in our survey yet. The survey allowed however to name alternative drugs and the application of a MEK inhibitor was indicated only twice by the respondents. The group of MEK inhibitors deserves specific attention in a possible follow-up survey in the future.
Although clinical response to BVZ is reported by the majority of respondents our survey did not extrapolate on the responses in vision and the differences between children with or without neurofibromatosis type 1.
Conclusion
Multiple different drug regimens are applied for progressive iCHG. Also in case of a neurosurgical approach subsequent anti-neoplastic treatment is widely applied. The application of targeted drugs emerges as novel treatment strategy. While vinblastine is the most common drug used in second line treatment, BVZ is often used in third line, mostly in combination with irinotecan. Prospective data on the efficacy of BVZ in LGG are needed and should include the infant population. Whereas the present findings cannot replace the lack of prospective trials for this small group of patients with progressive iCHG, they clearly reflect current strategies applied by an international community of paediatric neuro-oncologists. Still, treatment decisions have to be made on a patient per patient basis by an experienced interdisciplinary paediatric neuro-oncology team as depicted by the wide variety of possible therapeutic strategies. We advocate an international registry for children with (i)CHG to prospectively gather information on the multiple progressions and subsequent treatment strategies.
Abbreviations
- BVZ:
-
Bevacizumab
- CHG:
-
Chiasmatic hypothalamic glioma
- iCHG:
-
Infant chiasmatic hypothalamic glioma
- LGG:
-
Low-grade glioma
- SIOPE:
-
Societé Internationale d’Oncologie Pédiatrique in Europe
- TPCV:
-
Thioguanine, procarbazine, CCNU (1-(2-chloroethyl)-3-cyclohexyl-1-nitrosourea) and vincristine
- VEGF:
-
Vascular endothelial growth factor
References
Qaddoumi I, Sultan I, Gajjar A (2009) Outcome and prognostic features in pediatric gliomas: a review of 6212 cases from the surveillance, epidemiology, and end results database. Cancer 115:5761–5770
Garvey M, Packer RJ (1996) An integrated approach to the treatment of chiasmatic-hypothalamic gliomas. J Neurooncol 28:167–183
Mirow C, Pietsch T, Berkefeld S, Kwiecien R, Warmuth-Metz M, Falkenstein F, Diehl B, von Hornstein S, Gnekow AK (2014) Children < 1 year show an inferior outcome when treated according to the traditional LGG treatment strategy: a report from the German multicenter trial HIT-LGG 1996 for children with low grade glioma (LGG). Pediatr Blood Cancer 61:457–463
Stokland T, Liu JF, Ironside JW, Ellison DW, Taylor R, Robinson KJ, Picton SV, Walker DA (2010) A multivariate analysis of factors determining tumor progression in childhood low-grade glioma: a population-based cohort study (CCLG CNS9702). Neuro Oncol 12:1257–1268
Gnekow AK, Falkenstein F, von Hornstein S, Zwiener I, Berkefeld S, Bison B, Warmuth-Metz M, Driever PH, Soerensen N, Kortmann RD, Pietsch T, Faldum A (2012) Long-term follow-up of the multicenter, multidisciplinary treatment study HIT-LGG-1996 for low-grade glioma in children and adolescents of the German Speaking Society of Pediatric Oncology and Hematology. Neuro Oncol 14:1265–1284
Laithier V, Grill J, Le Deley MC, Ruchoux MM, Couanet D, Doz F, Pichon F, Rubie H, Frappaz D, Vannier JP, Babin-Boilletot A, Sariban E, Chastagner P, Zerah M, Raquin MA, Hartmann O, Kalifa C (2003) French Society of Pediatric O: progression-free survival in children with optic pathway tumors: dependence on age and the quality of the response to chemotherapy—results of the first French prospective study for the French Society of Pediatric Oncology. J Clin Oncol 21:4572–4578
Ahn Y, Cho BK, Kim SK, Chung YN, Lee CS, Kim IH, Yang SW, Kim HS, Kim HJ, Jung HW, Wang KC (2006) Optic pathway glioma: outcome and prognostic factors in a surgical series. Childs Nerv Syst 22:1136–1142
Hupp M, Falkenstein F, Bison B, Mirow C, Krauss J, Gnekow A, Solymosi L, Warmuth-Metz M (2012) Infarction following chiasmatic low grade glioma resection. Childs Nerv Syst 28:391–398
Krishnatry R, Zhukova N, Guerreiro Stucklin AS, Pole JD, Mistry M, Fried I, Ramaswamy V, Bartels U, Huang A, Laperriere N, Dirks P, Nathan PC, Greenberg M, Malkin D, Hawkins C, Bandopadhayay P, Kieran MW, Manley PE, Bouffet E, Tabori U (2016) Clinical and treatment factors determining long-term outcomes for adult survivors of childhood low-grade glioma: a population-based study. Cancer 122:1261–1269
Mulhern RK, Palmer SL, Merchant TE, Wallace D, Kocak M, Brouwers P, Krull K, Chintagumpala M, Stargatt R, Ashley DM, Tyc VL, Kun L, Boyett J, Gajjar A (2005) Neurocognitive consequences of risk-adapted therapy for childhood medulloblastoma. J Clin Oncol 23:5511–5519
de Ruiter MA, Schouten-van Meeteren AYN, van Vuurden DG, Maurice-Stam H, Gidding C, Beek LR, Granzen B, Oosterlaan J, Grootenhuis MA (2016) Psychosocial profile of pediatric brain tumor survivors with neurocognitive complaints. Qual Life Res 25:435–446
Packer RJ, Ater J, Allen J, Phillips P, Geyer R, Nicholson HS, Jakacki R, Kurczynski E, Needle M, Finlay J, Reaman G, Boyett JM (1997) Carboplatin and vincristine chemotherapy for children with newly diagnosed progressive low-grade gliomas. J Neurosurg 86:747–754
Gnekow AK, Walker DA, Kandels D, Picton S, Giorgio P, Grill J, Stokland T, Sandstrom PE, Warmuth-Metz M, Pietsch T, Giangaspero F, Schmidt R, Faldum A, Kilmartin D, De Paoli A, De Salvo GL, of the Low Grade Glioma C, the participating c (2017) A European randomised controlled trial of the addition of etoposide to standard vincristine and carboplatin induction as part of an 18-month treatment programme for childhood (≤ 16 years) low grade glioma: a final report. Eur J Cancer 81:206–225
de Haas V, Grill J, Raquin MA, Couanet D, Habrand JL, Sainte-Rose C, Laithier V, Kieffer V, Kalifa C (2009) Relapses of optic pathway tumors after first-line chemotherapy. Pediatr Blood Cancer 52:575–580
Scheinemann K, Bartels U, Tsangaris E, Hawkins C, Huang A, Dirks P, Fried I, Bouffet E, Tabori U (2011) Feasibility and efficacy of repeated chemotherapy for progressive pediatric low-grade gliomas. Pediatr Blood Cancer 57:84–88
Bouffet E, Jakacki R, Goldman S, Hargrave D, Hawkins C, Shroff M, Hukin J, Bartels U, Foreman N, Kellie S, Hilden J, Etzl M, Wilson B, Stephens D, Tabori U, Baruchel S (2012) Phase II study of weekly vinblastine in recurrent or refractory pediatric low-grade glioma. J Clin Oncol 30:1358–1363
Gururangan S, Fisher MJ, Allen JC, Herndon JE, 2nd, Quinn JA, Reardon DA, Vredenburgh JJ, Desjardins A, Phillips PC, Watral MA, Krauser JM, Friedman AH, Friedman HS: Temozolomide in children with progressive low-grade glioma. Neuro Oncol 9: 161–168, 2007
Soffietti R, Nobile M, Ruda R, Borgognone M, Costanza A, Laguzzi E, Mutani R (2004) Second-line treatment with carboplatin for recurrent or progressive oligodendroglial tumors after PCV (procarbazine, lomustine, and vincristine) chemotherapy: a phase II study. Cancer 100:807–813
Dodgshun AJ, Maixner WJ, Heath JA, Sullivan MJ, Hansford JR (2016) Single agent carboplatin for pediatric low-grade glioma: a retrospective analysis shows equivalent efficacy to multiagent chemotherapy. Int J Cancer 138:481–488
Packer RJ, Jakacki R, Horn M, Rood B, Vezina G, MacDonald T, Fisher MJ, Cohen B (2009) Objective response of multiply recurrent low-grade gliomas to bevacizumab and irinotecan. Pediatr Blood Cancer 52:791–795
Gururangan S, Fangusaro J, Poussaint TY, McLendon RE, Onar-Thomas A, Wu S, Packer RJ, Banerjee A, Gilbertson RJ, Fahey F, Vajapeyam S, Jakacki R, Gajjar A, Goldman S, Pollack IF, Friedman HS, Boyett JM, Fouladi M, Kun LE (2014) Efficacy of bevacizumab plus irinotecan in children with recurrent low-grade gliomas: a Pediatric Brain Tumor Consortium study. Neuro Oncol 16:310–317
Avery RA, Hwang EI, Jakacki RI, Packer RJ (2014) Marked recovery of vision in children with optic pathway gliomas treated with bevacizumab. JAMA Ophthalmol 132:111–114
Hwang EI, Jakacki RI, Fisher MJ, Kilburn LB, Horn M, Vezina G, Rood BR, Packer RJ (2013) Long-term efficacy and toxicity of bevacizumab-based therapy in children with recurrent low-grade gliomas. Pediatr Blood Cancer 60:776–782
Jakacki RI, Bouffet E, Adamson PC, Pollack IF, Ingle AM, Voss SD, Blaney SM (2011) A phase 1 study of vinblastine in combination with carboplatin for children with low-grade gliomas: a Children’s Oncology Group phase 1 consortium study. Neuro Oncol 13:910–915
Singh G, Wei XC, Hader W, Chan JA, Bouffet E, Lafay-Cousin L (2013) Sustained response to weekly vinblastine in 2 children with pilomyxoid astrocytoma associated with diencephalic syndrome. J Pediatr Hematol Oncol 35:e53-56
Heng MA, Padovani L, Dory-Lautrec P, Gentet JC, Verschuur A, Pasquier E, Figarella-Branger D, Scavarda D, Andre N (2016) Can metronomic maintenance with weekly vinblastine prevent early relapse/progression after bevacizumab-irinotecan in children with low-grade glioma? Cancer Med 5:1542–1545
Massimino M, Spreafico F, Cefalo G, Riccardi R, Tesoro-Tess JD, Gandola L, Riva D, Ruggiero A, Valentini L, Mazza E, Genitori L, Di Rocco C, Navarria P, Casanova M, Ferrari A, Luksch R, Terenziani M, Balestrini MR, Colosimo C, Fossati-Bellani F (2002) High response rate to cisplatin/etoposide regimen in childhood low-grade glioma. J Clin Oncol 20:4209–4216
Massimino M, Spreafico F, Riva D, Biassoni V, Poggi G, Solero C, Gandola L, Genitori L, Modena P, Simonetti F, Potepan P, Casanova M, Meazza C, Clerici CA, Catania S, Sardi I, Giangaspero F (2010) A lower-dose, lower-toxicity cisplatin-etoposide regimen for childhood progressive low-grade glioma. J Neurooncol 100:65–71
Djerassi I, Kim JS, Reggev A (1985) Response of astrocytoma to high-dose methotrexate with citrovorum factor rescue. Cancer 55:2741–2747
Rosenstock JG, Packer RJ, Bilaniuk L, Bruce DA, Radcliffe JL, Savino P (1985) Chiasmatic optic glioma treated with chemotherapy: a preliminary report. J Neurosurg 63:862–866
Janss AJ, Grundy R, Cnaan A, Savino PJ, Packer RJ, Zackai EH, Goldwein JW, Sutton LN, Radcliffe J, Molloy PT et al (1995) Optic pathway and hypothalamic/chiasmatic gliomas in children younger than age 5 years with a 6-year follow-up. Cancer 75:1051–1059
Kalra M, Heath JA, Kellie SJ, Dalla Pozza L, Stevens MM, Swamy S, McCowage GB (2015) Confirmation of bevacizumab activity, and maintenance of efficacy in retreatment after subsequent relapse, in pediatric low-grade glioma. J Pediatr Hematol Oncol 37:e341-346
Reismuller B, Azizi AA, Peyrl A, Heinrich M, Gruber-Olipitz M, Luckner D, Rothschild KV, Slavc I (2010) Feasibility and tolerability of bevacizumab in children with primary CNS tumors. Pediatr Blood Cancer 54:681–686
Fangusaro J, Gururangan S, Poussaint TY, McLendon RE, Onar-Thomas A, Warren KE, Wu S, Packer RJ, Banerjee A, Gilbertson RJ, Jakacki R, Gajjar A, Goldman S, Pollack IF, Friedman HS, Boyett JM, Kun LE, Fouladi M (2013) Bevacizumab (BVZ)-associated toxicities in children with recurrent central nervous system tumors treated with BVZ and irinotecan (CPT-11): a Pediatric Brain Tumor Consortium Study (PBTC-022). Cancer 119:4180–4187
Benesch M, Windelberg M, Sauseng W, Witt V, Fleischhack G, Lackner H, Gadner H, Bode U, Urban C (2008) Compassionate use of bevacizumab (Avastin) in children and young adults with refractory or recurrent solid tumors. Ann Oncol 19:807–813
Fangusaro J, Gururangan S, Jakacki RI, Kaste SC, Goldman S, Pollack IF, Boyett JM, Kun LE (2013) Bevacizumab-associated osteonecrosis of the wrist and knee in three pediatric patients with recurrent CNS tumors. J Clin Oncol 31:e24-27
Jones DT, Gronych J, Lichter P, Witt O, Pfister SM (2012) MAPK pathway activation in pilocytic astrocytoma. Cell Mol Life Sci 69:1799–1811
Karajannis MA, Legault G, Fisher MJ, Milla SS, Cohen KJ, Wisoff JH, Harter DH, Goldberg JD, Hochman T, Merkelson A, Bloom MC, Sievert AJ, Resnick AC, Dhall G, Jones DT, Korshunov A, Pfister SM, Eberhart CG, Zagzag D, Allen JC (2014) Phase II study of sorafenib in children with recurrent or progressive low-grade astrocytomas. Neuro Oncol 16:1408–1416
Banerjee A, Jakacki R, Onar-Thomas A, Wu SJ, Nicolaides T, Turner D, Richardson S, Young-Poussaint T, Phillips JJ, Prados M, Packer R, Qaddoumi IA, Gururangan S, Goldman S, Pollack I, Doyle LA, Stewart CF, Boyett JM, Fouladi M: A phase I study of AZD6244 in children with recurrent or refractory low-grade gliomas: a Pediatric Brain Tumor Consortium report. J Clin Oncol 32, 2014
Fangusaro J, Onar-Thomas A, Poussaint TY, Wu SJ, Ligon AH, Lindeman N, Banerjee A, Packer RJ, Kilburn LB, Pollack IF, Jakacki RI, Qaddoumi I, Fisher PG, Dhall G, Baxter P, Kreissman SG, Stewart CF, Pfister SM, Jones DTW, Vezina G, Stern J, Panigrahy A, Jones BV, Patay Z, Tamrazi B, Jones JY, Haque SS, Enterline DS, Cha S, Doyle LA, Smith M, Boyett JM, Dunkel IJ, Fouladi M (2017) A phase II prospective study of selumetinib in children with recurrent or refractory low-grade glioma (LGG): A Pediatric Brain Tumor Consortium (PBTC) study. Neuro Oncol 19:34–35
Benesch M, Lackner H, Sovinz P, Suppan E, Schwinger W, Eder HG, Dornbusch HJ, Moser A, Triebl-Roth K, Urban C (2006) Late sequela after treatment of childhood low-grade gliomas: a retrospective analysis of 69 long-term survivors treated between 1983 and 2003. J Neurooncol 78:199–205
Acknowledgement
Open access funding provided by Medical University of Vienna.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest concerning the contents of the present study. A. Azizi is member of the international Trial Steering Group of the HERBY study (clinicaltrials.gov NCT01390948) investigating the efficacy of bevacizumab in children with high grade glioma and his institution (the Medical University of Vienna) has received consultancy fees from F. Hoffmann-La Roche Ltd for attendance at HERBY Trial Steering Group meetings.
Data availability
The datasets analysed during the current study are available from the corresponding author on reasonable request.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Azizi, A.A., Schouten-van Meeteren, A.Y.N. Current and emerging treatment strategies for children with progressive chiasmatic-hypothalamic glioma diagnosed as infants: a web-based survey. J Neurooncol 136, 127–134 (2018). https://doi.org/10.1007/s11060-017-2630-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-017-2630-6