Abstract
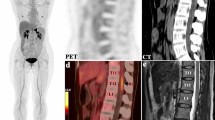
It is essential to be familiar with normal patterns of 18F FDG distribution in the whole body for accurate PET interpretation. We assessed FDG uptake by the spinal cord to evaluate its characteristics in cancer patients. For 101 cancer patients who underwent 18F FDG PET/CT the spinal cord along its segments was visually assessed for FDG uptake, regarding MaxSUV-measurement ≥1 as cut-off point. This assessment was correlated with the patient’s database variables. MRI and FDG PET-CT follow-up were included in the evaluation of positive subjects with FDG cord uptake. Forty-nine (48.5%) were positive for FDG cord uptake. The most encountered sites were the eleventh and twelfth dorsal vertebrae (36/49; 73.5%), all cervical (24/49; 49%), and the first lumbar segments (19/49; 38.7%). 38/49 (77.6%) and 11/49 (22.4%) were detected in the winter and summer, respectively (P = 0.007). MRI was available for 25 of the positive FDG cord uptake patients and showed no cord abnormalities, and in follow-up FDG PET-CT studies within 3–6 months 41/49 (83.7%) faded completely, while stationary or reduced uptake was observed for the remainder (8/49; 16.3%). FDG uptake in multiple consecutive segments of the spinal cord is not uncommon in cancer patients. This must be recognized as physiological, to avoid misdiagnosis as malignant involvement. Such physiological uptake is mostly encountered in the cervical, last two dorsal, and first lumbar levels, and quite frequently in winter.




Similar content being viewed by others
References
Goerres GW, Ziegler SI, Burger C et al (2003) Artifacts at PET and PET/CT caused by metallic hip prosthetic material. Radiology 226:577–584
Bar-Shalom R, Valdivia AY, Blaufox MD (2000) PET imaging in oncology. Semin Nucl Med 30:150–185
Bomanji JB, Costa DC, Ell PJ (2001) Clinical role of positron emission tomography in oncology. Lancet Oncol 2:157–164
Hustinx R, Benard F, Alavi A (2002) Whole-body FDG-PET imaging in the management of patients with cancer. Semin Nucl Med 32:35–46
Nakamoto Y, Tatsumi M, Hammoud D et al (2005) Normal FDG distribution patterns in the head and neck: PET/CT evaluation. Radiology 234:879–885
McDermott S, Skehan SJ (2010) Whole body imaging in the abdominal cancer patient: pitfalls of PET-CT. Abdom Imaging 35:55–69 (review)
Do BH, Mari C, Tseng JR, Quon A, Rosenberg J, Biswal S (2001) Pattern of 18F-FDG uptake in the spinal cord in patients with non-central nervous system malignancy. Spine (Phila Pa 1976) 36:E1395–E1401
Metser U, Lerman H, Blank A et al (2004) Malignant involvement of the spine: assessment by 18F-FDG PET/CT. J Nucl Med 45:279–284
Floeth FW, Stoffels G, Herdmann J et al (2010) Regional impairment of 18F-FDG uptake in the cervical spinal cord in patients with monosegmental chronic cervical myelopathy. Eur Radiol 20:2925–2932
Floeth FW, Stoffels G, Herdmann J et al (2011) Prognostic value of 18F-FDG PET in monosegmental stenosis and myelopathy of the cervical spinal cord. J Nucl Med 52:1385–1391
Kadrmas DJ, Casey ME, Black NF, Hamill JJ, Panin VY, Conti M (2009) Experimental comparison of lesion detectability for four fully-3D PET reconstruction schemes. IEEE Trans Med Imaging 28:523–534
Alessio AM, Kinahan PE, Lewellen TK (2006) Modeling and incorporation of system response functions in 3-D whole body PET. IEEE Trans Med Imaging 25:828–837
Panin VY, Kehren F, Michel C, Casey M (2006) Fully-3-D PET reconstruction with system matrix derived from point source measurements. IEEE Trans Med Imaging 25:907–921
Esik O, Emri M, Szakáll S Jr et al (2004) PET identifies transitional metabolic change in the spinal cord following a subthreshold dose of irradiation. Pathol Oncol Res 10:42–46
Kamoto Y, Sadato N, Yonekura Y et al (1998) Visualization of the cervical spinal cord with FDG and high-resolution PET. J Comput Assist Tomogr 22:487–491
Uematsu H, Sadato N, Yonekura Y et al (1998) Coregistration of FDG PET and MRI of the head and neck using normal distribution of FDG. J Nucl Med 39:2121–2127
Gray H (1989) Spinal medulla or cord. In: William PL (ed) Gray’s anatomy, 37th edn. Churchill Livingstone, New York, p 922
Schafer EA (1990) The spinal cord and brain. In: Quain J, Schafer EA, Thane GD (eds) Quain’s elements of anatomy. Longmans, Green and Co., London, pp 1–219
Kameyama T, Hashizume Y, Sobue G (1996) Morphologic features of the normal human cadaveric spinal cord. Spine 21:1285–1290
Kadrmas DJ (2004) LOR-OSEM: statistical PET reconstruction from raw line-of-response histograms. Phys Med Biol 49:4731–4744
Kadrmas DJ (2008) Rotate-and-slant projector for fast LOR-based fully-3-D iterative PET reconstruction. IEEE Trans Med Imaging 27:1071–1083
Fehm HL, Kern W, Peters A (2006) The selfish brain: competition for energy resources. Prog Brain Res 153:129–140
Jadvar H, Connolly LP, Fahey FH, Shulkin BL (2007) PET and PET/CT in pediatric oncology. Semin Nucl Med 37:316–331
Kawashita NH, Brito MN, Brito SR et al (2002) Glucose uptake, glucose transporter GLUT4, and glycolytic enzymes in brown adipose tissue from rats adapted to a high-protein diet. Metabolism 51:1501–1505
Yeung HW, Grewal RK, Gonen M, Schoder H, Larsen SM (2003) Patterns of (18)F-FDG uptake in adipose tissue and muscle: a potential source of false-positives for PET. J Nucl Med 44:1789–1796
Shammas A, Lim R, Charron M (2009) Pediatric FDG PET/CT: physiologic uptake, normal variants, and benign conditions. Radiographics 29:1467–1486
Karantanis D, O’eill BP, Subramaniam RM et al (2007) 18F-FDG PET/CT in primary central nervous system lymphoma in HIV-negative patients. Nucl Med Commun 28:834–841
Nguyen NC, Sayed MM, Taalab K, Osman MM (2008) Spinal cord metastases from lung cancer: detection with F-18 FDG PET/CT. Clin Nucl Med 33:356–358
Acknowledgment
The authors thank Dr Zeinab Nawito for her helpful contributions in the finalization of this study regarding grammatical aspects.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Amin, A., Rosenbaum, S.J. & Bockisch, A. Physiological 18F-FDG uptake by the spinal cord: is it a point of consideration for cancer patients?. J Neurooncol 107, 609–615 (2012). https://doi.org/10.1007/s11060-011-0785-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-011-0785-0