Abstract
Objective
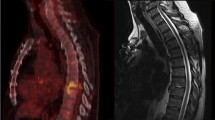
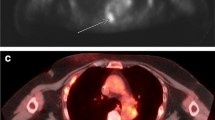
In positron emission tomography (PET) with F-18-fluorodeoxyglucose (FDG), non-tumorous focal uptake is often observed around the lumbar spinous processes (LSPs). Close approximation of LSPs with sclerosis is often seen, which is called Baastrup disease. The aim of this study was to characterize this finding in terms of location and subjects’ age and investigate the relation between PET and CT findings.
Methods
The PET/CT scans of 40 patients each in the fifth, sixth, seventh, eighth, and ninth decades were screened for FDG uptake around the LSPs from L1–2 through L5–S1. Patients with metastasis to the lumbar spine or recent chemotherapy or rheumatoid arthritis-related disease were excluded. Focal uptake greater than blood pool activity was considered positive. Positive uptake was compared among the ages and locations. We also evaluated the relationship between FDG uptake and CT morphology.
Results
Overall, focal uptake was observed in 122 LSPs in 71 patients. At least one positive uptake was seen in 9, 21, 15, 12, and 14 of 40 patients (16, 30, 30, 20, and 26 of 200 regions) in each age group of 40s through 80s, respectively (p = 0.12). As for the location, uptake around L1–2, L2–3, L3–4, L4–5, and L5–S1 was observed in 19, 22, 39, 35, and 7 regions, respectively (p < 0.01). There was no statistically significant difference in PET positivity among the five age groups, but positive uptake was predominantly seen in L3–4. Degeneration on CT was apparent in 58, 74, 108, 123, and 144 regions in each age group, respectively (p < 0.01), and in 38, 79, 131, 151, and 108 regions in each location, respectively (p < 0.01). The PET positive ratio was higher in CT positive group than in CT negative group (14 vs. 10 %, p < 0.05), but there was no significant difference of quantitative values (p = 0.10). Of 42 regions in 27 patients who had serial PET/CT scans that were initially PET-positive, 35 regions (83 %) turned negative on a later PET-scan.
Conclusions
Focal uptake around the LSPs was commonly seen in the mid-lumbar vertebrae, independent of age, and was not always correlated with morphological changes. This uptake should not be assumed to represent osseous metastasis.



Similar content being viewed by others
References
Boellaard R. Standards for PET image acquisition and quantitative data analysis. J Nucl Med. 2009;50:11S–20S.
Zincirkeser S, Sahin E, Halac M, Sager S. Standardized uptake values of normal organs on 18F-fluorodeoxyglucose positron emission tomography and computed tomography imaging. J Int Med Res. 2007;35:231–6.
Rosen RS, Fayad L, Wahl RL. Increased 18F-FDG uptake in degenerative disease of the spine: characterization with 18F-FDG PET/CT. J Nucl Med. 2006;47:1274–80.
Stumpe KD, Zanetti M, Weishaupt D, Hodler J, Boos N, Von Schulthess GK. FDG positron emission tomography for differentiation of degenerative and infectious endplate abnormalities in the lumbar spine detected on MR imaging. Am J Roentgenol. 2002;179:1151–7.
Yamashita H, Kubota K, Takahashi Y, Minamimoto R, Morooka M, Kaneko H, et al. Similarities and differences in fluorodeoxyglucose positron emission tomography/computed tomography findings in spondyloarthropathy, polymyalgia rheumatica and rheumatoid arthritis. Joint Bone Spine. 2013;80:171–7.
Baastrup CI. On the spinous processes of the lumbar vertebrae and the soft tissues between them, and on pathological changes in that region. Acta Radiol. 1933;14:52–5.
Resnick D. Degenerative diseases of the vertebral column. Radiology. 1985;156:3–14.
Beks JW. Kissing spines: fact or fancy? Acta Neurochir. 1989;100:134–5.
Sartoris DJ, Resnick D, Tyson R, Haghighi P. Age-related alterations in the vertebral spinous processes and intervening soft tissues: radiologic-pathologic correlation. Am J Roentgenol. 1985;145:1025–30.
Kwong Y, Rao N, Latief K. MDCT findings in Baastrup disease: disease or normal feature of the aging spine? Am J Roentgenol. 2011;196:1156–9.
Aylott CE, Puna R, Robertson PA, Walker C. Spinous process morphology: the effect of ageing through adulthood on spinous process size and relationship to sagittal alignment. Eur Spine J. 2012;21:1007–12.
Maes R, Morrison WB, Parker L, Schweitzer ME, Carrino JA. Lumbar interspinous bursitis (Baastrup disease) in a symptomatic population: prevalence on magnetic resonance imaging. Spine. 2008;33:E211–5.
Hui C, Cox I. Two unusual presentations of Baastrup’s disease. Clin Radiol. 2007;62:495–7.
Jang EC, Song KS, Lee HJ, Kim JY, Yang JJ. Posterior epidural fibrotic mass associated with Baastrup’s disease. Eur Spine J. 2010;19:S165–8.
Chen CK, Yeh L, Resnick D, Lai PH, Liang HL, Pan HB, et al. Intraspinal posterior epidural cysts associated with Baastrup’s disease: report of 10 patients. Am J Roentgenol. 2004;182:191–4.
Keorochana G, Taghavi CE, Tzeng ST, Lee KB, Liao JC, Yoo JH, et al. MRI classification of interspinous ligament degeneration of the lumbar spine: intraobserver and interobserver reliability and the frequency of disagreement. Eur Spine J. 2010;19:1740–5.
Ho L, Wassef H, Seto J, Henderson R. Multi-level lumbar baastrup disease on F-18 FDG PET–CT. Clin Nucl Med. 2009;34:896–7.
Lin E. Baastrup’s disease (kissing spine) demonstrated by FDG PET/CT. Skelet Radiol. 2008;37:173–5.
Gorospe L, Jover R, Vicente-Bártulos A, González-Gordaliza C, de Llanos CG, García-Poza J. FDG-PET/CT demonstration of Baastrup disease (“Kissing” Spine). Clin Nucl Med. 2008;33:133–4.
Aliyev A, Saboury B, Kwee TC, Torigian DA, Basu S, Wulff Christensen H, et al. Age-related inflammatory changes in the spine as demonstrated by 18F-FDG-PET: observation and insight into degenerative spinal changes. Hell J Nucl Med. 2012;15:197–201.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nishimatsu, K., Nakamoto, Y., Ishimori, T. et al. FDG uptake observed around the lumbar spinous process: relevance to Baastrup disease. Ann Nucl Med 29, 766–771 (2015). https://doi.org/10.1007/s12149-015-1003-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12149-015-1003-5