Abstract
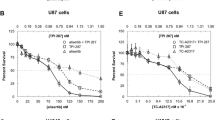
The main problem in the treatment of malignant astrocytomas is their invasive behaviour. Successful resection of the main tumour mass cannot prevent recurrence due to single cells invading the surrounding brain parenchyma at the time of diagnosis. The classical combination therapy, PCV (Procarbazine, CCNU and Vincristine) used for over 30 years; has shown its clinical effectiveness in the treatment of malignant astrocytomas and glioblastomas is still doubtful. Using an in vitro three dimensional invasion model, we tested the effect of the tyrosine kinase inhibitor imatinib and the microtubule inhibitor docetaxel on the invasion activity of a panel of astrocytic tumour cell lines, including two established glioma cell lines, IPSB-18 and SNB-19, and two primary cell lines, originating from glioblastomas, CLOM002 and UPHHJA, and in normal astrocytes. A dose response curve for each drug alone and in combination was determined. The half maximal inhibitory concentration (IC50) concentration of imatinib was between 15.7 and 18.7 μM, which did not affect invasion activity of the cell lines. The IC50 concentration of docetaxel was between 0.7 and 19.8 nM, and at 14.9 nM docetaxel had a slight transient inhibitory effect on invasion activity of all tested cells. The combination of imatinib at 13.5 μM and docetaxel at 14.9 nM, however, synergistically inhibited cell growth and invasion activity and could not be reversed by drug removal. A combination treatment with tyrosine kinase inhibitors and cytotoxic drugs shows promise in tackling both glioma proliferation and invasion, and could present a new treatment regimen for malignant astrocytomas.








Similar content being viewed by others
References
Stupp R et al (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352(10):987–996
Ohgaki H et al (2004) Genetic pathways to glioblastoma: a population-based study. Cancer Res 64(19):6892–6899
Schlessinger J (2000) Cell signaling by receptor tyrosine kinases. Cell 103(2):211–225
Kleihues P et al (2002) The WHO classification of tumors of the nervous system. J Neuropathol Exp Neurol 61(3):215–225 discussion 226-9
Hermanson M et al (1992) Platelet-derived growth factor and its receptors in human glioma tissue: expression of messenger RNA and protein suggests the presence of autocrine and paracrine loops. Cancer Res 52(11):3213–3219
Westermark B, Heldin CH, Nister M (1995) Platelet-derived growth factor in human glioma. Glia 15(3):257–263
Sherbenou DW, Druker BJ (2007) Applying the discovery of the Philadelphia chromosome. J Clin Invest 117(8):2067–2074
Kilic T et al (2000) Intracranial inhibition of platelet-derived growth factor-mediated glioblastoma cell growth by an orally active kinase inhibitor of the 2-phenylaminopyrimidine class. Cancer Res 60(18):5143–5150
Nagar B (2007) c-Abl tyrosine kinase and inhibition by the cancer drug imatinib (Gleevec/STI-571). J Nutr 137(6 Suppl 1):1518S–1523S discussion 1548S
Raymond E et al (2008) Phase II study of imatinib in patients with recurrent gliomas of various histologies: a European Organisation for Research and Treatment of Cancer Brain Tumor Group Study. J Clin Oncol 26(28):4659–4665
Katz D et al (2004) Neoadjuvant imatinib for unresectable gastrointestinal stromal tumor. Anticancer Drugs 15(6):599–602
Hagerstrand D et al (2006) Characterization of an imatinib-sensitive subset of high-grade human glioma cultures. Oncogene 25(35):4913–4922
Bissery MC, et al. (1995) Docetaxel (Taxotere): a review of preclinical and clinical experience. Part I: preclinical experience. Anticancer Drugs 6(3):339–355, 363–368
Plosker GL, Hurst M (2001) Paclitaxel: a pharmacoeconomic review of its use in non-small cell lung cancer. Pharmacoeconomics 19(11):1111–1134
Sanson M et al (2000) Second line chemotherapy with docetaxel in patients with recurrent malignant glioma: a phase II study. J Neurooncol 50(3):245–249
Forsyth P et al (1996) Phase II trial of docetaxel in patients with recurrent malignant glioma: a study of the National Cancer Institute of Canada Clinical Trials Group. Invest New Drugs 14(2):203–206
Koukourakis MI et al (1999) Concurrent twice-a-week docetaxel and radiotherapy: a dose escalation trial with immunological toxicity evaluation. Int J Radiat Oncol Biol Phys 43(1):107–114
Sampath P et al (2006) Interstitial docetaxel (Taxotere), carmustine and combined interstitial therapy: a novel treatment for experimental malignant glioma. J Neurooncol 80(1):9–17
Heenan M et al (1997) Isolation from a human MDR lung cell line of multiple clonal subpopulations which exhibit significantly different drug resistance. Int J Cancer 71(5):907–915
Chou TC, Talalay P (1984) Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul 22:27–55
Towbin H, Staehelin T, Gordon J (1979) Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA 76(9):4350–4354
Del Duca D, Werbowetski T, Del Maestro RF (2004) Spheroid preparation from hanging drops: characterization of a model of brain tumor invasion. J Neurooncol 67(3):295–303
Servidei T et al (2006) Increased sensitivity to the platelet-derived growth factor (PDGF) receptor inhibitor STI571 in chemoresistant glioma cells is associated with enhanced PDGF-BB-mediated signaling and STI571-induced Akt inactivation. J Cell Physiol 208(1):220–228
Gucluler G, Baran Y (2009) Docetaxel enhances the cytotoxic effects of imatinib on Philadelphia positive human chronic myeloid leukemia cells. Hematology 14(3):139–144
Amberger VR et al (1997) Oligodendrocyte-type 2 astrocyte progenitors use a metalloendoprotease to spread and migrate on CNS myelin. Eur J Neurosci 9(1):151–162
Ren H et al (2009) Differential effect of imatinib and synergism of combination treatment with chemotherapeutic agents in malignant glioma cells. Basic Clin Pharmacol Toxicol 104(3):241–252
Bihorel S et al (2007) Influence of breast cancer resistance protein (Abcg2) and p-glycoprotein (Abcb1a) on the transport of imatinib mesylate (Gleevec) across the mouse blood-brain barrier. J Neurochem 102(6):1749–1757
le Coutre P et al (2004) Pharmacokinetics and cellular uptake of imatinib and its main metabolite CGP74588. Cancer Chemother Pharmacol 53(4):313–323
Kemper EM et al (2003) Increased penetration of paclitaxel into the brain by inhibition of P-Glycoprotein. Clin Cancer Res 9(7):2849–2855
Kemper EM et al (2004) Improved penetration of docetaxel into the brain by co-administration of inhibitors of P-glycoprotein. Eur J Cancer 40(8):1269–1274
Fracasso PM et al (2004) Phase I study of docetaxel in combination with the P-glycoprotein inhibitor, zosuquidar, in resistant malignancies. Clin Cancer Res 10(21):7220–7228
Chamberlain MC (2006) Treatment options for glioblastoma. Neurosurg Focus 20(4):E2–E55
Carella AM, et al. (2010) Kinase domain mutations of BCR-ABL identified at diagnosis before imatinib-based therapy are associated with progression in patients with high Sokal risk chronic phase chronic myeloid leukemia. Leuk Lymphoma 51(2):275–278
Kim SH, et al. (2009) Analysis of Bcr-Abl kinase domain mutations in Korean chronic myeloid leukaemia patients: poor clinical outcome of P-loop and T315I mutation is disease phase dependent. Hematol Oncol 27(4):190–197
Hynes NE, Lane HA (2005) ERBB receptors and cancer: the complexity of targeted inhibitors. Nat Rev Cancer 5(5):341–354
Heimberger AB et al (2005) Prognostic effect of epidermal growth factor receptor and EGFRvIII in glioblastoma multiforme patients. Clin Cancer Res 11(4):1462–1466
Haberler C et al (2006) Immunohistochemical analysis of platelet-derived growth factor receptor-alpha, -beta, c-kit, c-abl, and arg proteins in glioblastoma: possible implications for patient selection for imatinib mesylate therapy. J Neurooncol 76(2):105–109
Jiang R et al (2006) Pathway alterations during glioma progression revealed by reverse phase protein lysate arrays. Proteomics 6(10):2964–2971
Stanulla M et al (1995) Coexpression of stem cell factor and its receptor c-Kit in human malignant glioma cell lines. Acta Neuropathol 89(2):158–165
Steelman LS et al (2004) JAK/STAT, Raf/MEK/ERK, PI3K/Akt and BCR-ABL in cell cycle progression and leukemogenesis. Leukemia 18(2):189–218
Skorski T et al (1995) Phosphatidylinositol-3 kinase activity is regulated by BCR/ABL and is required for the growth of Philadelphia chromosome-positive cells. Blood 86(2):726–736
Mogi M et al (2003) Akt signaling regulates side population cell phenotype via Bcrp1 translocation. J Biol Chem 278(40):39068–39075
Leslie EM, Deeley RG, Cole SP (2005) Multidrug resistance proteins: role of P-glycoprotein, MRP1, MRP2, and BCRP (ABCG2) in tissue defense. Toxicol Appl Pharmacol 204(3):216–237
van den Heuvel-Eibrink MM et al (2007) CD34-related coexpression of MDR1 and BCRP indicates a clinically resistant phenotype in patients with acute myeloid leukemia (AML) of older age. Ann Hematol 86(5):329–337
Acknowledgements
We would like to sincerely thank Prof. Geoff Pilkington, university of Portsmouth, for his kind donation of the cell lines CLOM002, UPHHJA and IPSB-18. We would also like to thank Cancer Research Ireland for their support in carrying out this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kinsella, P., Clynes, M. & Amberger-Murphy, V. Imatinib and docetaxel in combination can effectively inhibit glioma invasion in an in vitro 3D invasion assay. J Neurooncol 101, 189–198 (2011). https://doi.org/10.1007/s11060-010-0246-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-010-0246-1