Abstract
Introduction
Glioblastoma (GBM) has a very poor prognosis despite current treatment. We previously found cytotoxic synergy between the AURKA inhibitor alisertib and the CNS-penetrating taxane TPI 287 against GBM tumor cells in vitro.
Methods
We used an orthotopic human GBM xenograft mouse model to test if TPI 287 potentiates alisertib in vivo. Western blotting, immunohistochemistry, siRNA knockdown, annexin V binding, and 3-dimensional Matrigel invasion assays were used to investigate potential mechanisms of alisertib and TPI 287 treatment interactions.
Results
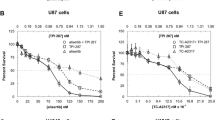
Alisertib + TPI 287 combination therapy significantly prolonged animal survival compared to vehicle (p = 0.011), but only marginally compared to alisertib alone. Alisertib, TPI 287, and combined alisertib + TPI 287 reduced animal tumor volume compared to vehicle-treated controls. This was statistically significant for the combination therapy at 4 weeks (p < 0.0001). Alisertib + TPI 287 treatment decreased anti-apoptotic Bcl-2 protein levels in vivo and in vitro. Expression of the pro-apoptotic protein Bak was significantly increased by combination treatment (p < 0.0001). Pro-apoptotic Bim and Bak knockdown by siRNA decreased apoptosis by alisertib + TPI 287 in GB9, GB30, and U87 cells (p = 0.0005 to 0.0381). Although alisertib and TPI 287 significantly reduced GBM cell invasion (p < 0.0001), their combination was no more effective than TPI 287 alone.
Conclusions
Results suggest that apoptosis is the dominant mechanism of potentiation of GBM growth inhibition by alisertib + TPI 287, in part through effects on Bcl-2 family proteins, providing a rationale for further laboratory testing of an AURKA inhibitor plus TPI 287 as a potential therapy against GBM.





Similar content being viewed by others
Data availability
All raw data will be provided by the authors upon reasonable request.
References
Ostrom QT, Cioffi G, Gittleman H, Patil N, Waite K, Kruchko C, Barnholtz-Sloan JS (2019) CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in. Neuro Oncol 21(5):1–100
Barr AR, Gergely F (2007) Aurora-A: the maker and breaker of spindle poles. J Cell Sci 120(Pt 17):2987–2996. https://doi.org/10.1242/jcs.013136
Lehman NL, O’Donnell JP, Whiteley LJ, Stapp RT, Lehman TD, Roszka KM, Schultz LR, Williams CJ, Mikkelsen T, Brown SL, Ecsedy JA, Poisson LM (2012) Aurora A is differentially expressed in gliomas, is associated with patient survival in glioblastoma and is a potential chemotherapeutic target in gliomas. Cell Cycle 11(3):489–502. https://doi.org/10.4161/cc.11.3.18996
Qiao W, Guo B, Zhou H, Xu W, Chen Y, Liang Y, Dong B (2017) miR-124 suppresses glioblastoma growth and potentiates chemosensitivity by inhibiting AURKA. Biochem Biophys Res Commun 486(1):43–48. https://doi.org/10.1016/j.bbrc.2017.02.120
Asteriti IA, Giubettini M, Lavia P, Guarguaglini G (2011) Aurora-A inactivation causes mitotic spindle pole fragmentation by unbalancing microtubule-generated forces. Mol Cancer 10:131. https://doi.org/10.1186/1476-4598-10-131
Zumbar CT, Usubalieva A, King PD, Li X, Mifsud CS, Dalton HM, Sak M, Urio S, Bryant WM, McElroy JP, Farmer G, Lehman NL (2018) The CNS penetrating taxane TPI 287 and the AURKA inhibitor alisertib induce synergistic apoptosis in glioblastoma cells. J Neurooncol 137(3):481–492. https://doi.org/10.1007/s11060-018-2755-2
Sak M, Zumbar CT, King PD, Li X, Mifsud CS, Usubalieva A, Anderson CD, Chesnick HM, McElroy JP, Chakravarti A, Burton EC, Lehman NL (2019) Cytotoxic synergy between alisertib and carboplatin versus alisertib and irinotecan are inversely dependent on MGMT levels in glioblastoma cells. J Neurooncol. https://doi.org/10.1007/s11060-019-03164-5
Van Brocklyn JR, Wojton J, Meisen WH, Kellough DA, Ecsedy JA, Kaur B, Lehman NL (2014) Aurora-A inhibition offers a novel therapy effective against intracranial glioblastoma. Cancer Res 74(19):5364–5370. https://doi.org/10.1158/0008-5472.CAN-14-0386
Hong X, O’Donnell JP, Salazar CR, Van Brocklyn JR, Barnett KD, Pearl DK, deCarvalho AC, Ecsedy JA, Brown SL, Mikkelsen T, Lehman NL (2014) The selective Aurora-A kinase inhibitor MLN8237 (alisertib) potently inhibits proliferation of glioblastoma neurosphere tumor stem-like cells and potentiates the effects of temozolomide and ionizing radiation. Cancer Chemother Pharmacol 73(5):983–990. https://doi.org/10.1007/s00280-014-2430-z
Zou Z, Yuan Z, Zhang Q, Long Z, Chen J, Tang Z, Zhu Y, Chen S, Xu J, Yan M, Wang J, Liu Q (2012) Aurora kinase A inhibition-induced autophagy triggers drug resistance in breast cancer cells. Autophagy 8(12):1798–1810. https://doi.org/10.4161/auto.22110
Heimans JJ, Vermorken JB, Wolbers JG, Eeltink CM, Meijer OW, Taphoorn MJ, Beijnen JH (1994) Paclitaxel (Taxol) concentrations in brain tumor tissue. Ann Oncol 5(10):951–953. https://doi.org/10.1093/oxfordjournals.annonc.a058736
Fitzgerald DP, Emerson DL, Qian Y, Anwar T, Liewehr DJ, Steinberg SM, Silberman S, Palmieri D, Steeg PS (2012) TPI-287, a new taxane family member, reduces the brain metastatic colonization of breast cancer cells. Mol Cancer Ther 11(9):1959–1967. https://doi.org/10.1158/1535-7163.MCT-12-0061
Tsujimoto Y (1998) Role of Bcl-2 family proteins in apoptosis: apoptosomes or mitochondria? Genes Cells 3(11):697–707. https://doi.org/10.1046/j.1365-2443.1998.00223.x
Williams B, Zumbar C, Sak M, Lehman N (2020) EXTH-22 The CNS penetrating taxane TPI 287 and the AURKA inhibitor alisertib improve survival in vivo. Neuro Oncol 22(2):91–91
Chen J, Ananthanarayanan B, Springer KS, Wolf KJ, Sheyman SM, Tran VD, Kumar S (2020) Suppression of LIM kinase 1 and LIM kinase 2 limits glioblastoma invasion. Cancer Res 80(1):69–78. https://doi.org/10.1158/0008-5472.CAN-19-1237
Vermeulen K, Berneman ZN, Van Bockstaele DR (2003) Cell cycle and apoptosis. Cell Prolif 36(3):165–175. https://doi.org/10.1046/j.1365-2184.2003.00267.x
Lu Z, Miao Y, Muhammad I, Tian E, Hu W, Wang J, Wang B, Li R, Li J (2017) Colistin-induced autophagy and apoptosis involves the JNK-Bcl2-Bax signaling pathway and JNK-p53-ROS positive feedback loop in PC-12 cells. Chem Biol Interact 277:62–73. https://doi.org/10.1016/j.cbi.2017.08.011
Kim H, Watanabe S, Kitamatsu M, Watanabe K, Ohtsuki T (2020) Cell cycle dependence of apoptosis photo-triggered using peptide-photosensitizer conjugate. Sci Rep 10(1):19087. https://doi.org/10.1038/s41598-020-76100-7
Katayama H, Wang J, Treekitkarnmongkol W, Kawai H, Sasai K, Zhang H, Wang H, Adams HP, Jiang S, Chakraborty SN, Suzuki F, Arlinghaus RB, Liu J, Mobley JA, Grizzle WE, Wang H, Sen S (2012) Aurora kinase-A inactivates DNA damage-induced apoptosis and spindle assembly checkpoint response functions of p73. Cancer Cell 21(2):196–211. https://doi.org/10.1016/j.ccr.2011.12.025
Vilgelm AE, Pawlikowski JS, Liu Y, Hawkins OE, Davis TA, Smith J, Weller KP, Horton LW, McClain CM, Ayers GD, Turner DC, Essaka DC, Stewart CF, Sosman JA, Kelley MC, Ecsedy JA, Johnston JN, Richmond A (2015) MDM2 and Aurora kinase A inhibitors synergize to block melanoma growth by driving apoptosis and immune clearance of tumor cells. Cancer Res 75(1):181–193. https://doi.org/10.1158/0008-5472.CAN-14-2405
Hemann MT, Lowe SW (2006) The p53-Bcl-2 connection. Cell Death Differ 13(8):1256–1259. https://doi.org/10.1038/sj.cdd.4401962
Sasai K, Treekitkarnmongkol W, Kai K, Katayama H, Sen S (2016) Functional significance of Aurora kinases-p53 protein family interactions in cancer. Front Oncol 6:247. https://doi.org/10.3389/fonc.2016.00247
Huang XF, Luo SK, Xu J, Li J, Xu DR, Wang LH, Yan M, Wang XR, Wan XB, Zheng FM, Zeng YX, Liu Q (2008) Aurora kinase inhibitory VX-680 increases Bax/Bcl-2 ratio and induces apoptosis in Aurora-A-high acute myeloid leukemia. Blood 111(5):2854–2865. https://doi.org/10.1182/blood-2007-07-099325
Hou D, Che Z, Chen P, Zhang W, Chu Y, Yang D, Liu J (2018) Suppression of AURKA alleviates p27 inhibition on Bax cleavage and induces more intensive apoptosis in gastric cancer. Cell Death Dis 9(8):781. https://doi.org/10.1038/s41419-018-0823-3
Ciechomska IA, Gielniewski B, Wojtas B, Kaminska B, Mieczkowski J (2020) EGFR/FOXO3a/BIM signaling pathway determines chemosensitivity of BMP4-differentiated glioma stem cells to temozolomide. Exp Mol Med 52(8):1326–1340. https://doi.org/10.1038/s12276-020-0479-9
Guan H, Song L, Cai J, Huang Y, Wu J, Yuan J, Li J, Li M (2011) Sphingosine kinase 1 regulates the Akt/FOXO3a/Bim pathway and contributes to apoptosis resistance in glioma cells. PLoS ONE 6(5):19946
Moustafa-Kamal M, Gamache I, Lu Y, Li S, Teodoro JG (2013) BimEL is phosphorylated at mitosis by Aurora A and targeted for degradation by betaTrCP1. Cell Death Differ 20(10):1393–1403. https://doi.org/10.1038/cdd.2013.93
Sunters A, Fernandez de Mattos S, Stahl M, Brosens JJ, Zoumpoulidou G, Saunders CA, Coffer PJ, Medema RH, Coombes RC, Lam EW (2003) FoxO3a transcriptional regulation of Bim controls apoptosis in paclitaxel-treated breast cancer cell lines. J Biol Chem 278(50):49795–49805. https://doi.org/10.1074/jbc.M309523200
Cartron PF, Loussouarn D, Campone M, Martin SA, Vallette FM (2012) Prognostic impact of the expression/phosphorylation of the BH3-only proteins of the BCL-2 family in glioblastoma multiforme. Cell Death Dis 3:421
Rieger L, Weller M, Bornemann A, Schabet M, Dichgans J, Meyermann R (1998) BCL-2 family protein expression in human malignant glioma: a clinical-pathological correlative study. J Neurol Sci 155(1):68–75. https://doi.org/10.1016/s0022-510x(97)00277-3
Xia JL, Fan WJ, Zheng FM, Zhang WW, Xie JJ, Yang MY, Kamran M, Wang P, Teng HM, Wang CL, Liu Q (2017) Inhibition of AURKA kinase activity suppresses collective invasion in a microfluidic cell culture platform. Sci Rep 7(1):2973. https://doi.org/10.1038/s41598-017-02623-1
Chen C, Song G, Xiang J, Zhang H, Zhao S, Zhan Y (2017) AURKA promotes cancer metastasis by regulating epithelial-mesenchymal transition and cancer stem cell properties in hepatocellular carcinoma. Biochem Biophys Res Commun 486(2):514–520. https://doi.org/10.1016/j.bbrc.2017.03.075
Boiarska Z, Passarella D (2021) Microtubule-targeting agents and neurodegeneration. Drug Discov Today 26(2):604–615. https://doi.org/10.1016/j.drudis.2020.11.033
Mitchell D, Bergendahl G, Ferguson W, Roberts W, Higgins T, Ashikaga T, DeSarno M, Kaplan J, Kraveka J, Eslin D, Werff AV, Hanna GK, Sholler GL (2016) A Phase 1 trial of TPI 287 as a single agent and in combination with temozolomide in patients with refractory or recurrent neuroblastoma or medulloblastoma. Pediatr Blood Cancer 63(1):39–46. https://doi.org/10.1002/pbc.25687
Cortice Biosciences Final results from the dose-escalation stage of a phase 1/2 trial of TPI 287, a brain penetrable microtubule inhibitor, plus bevacizumab in patients with recurrent glioblastoma. http://meetinglibrary.asco.org/record/146708/abstract. ASCO Meeting Library. Accessed date 25 May 2017
MD Anderson Cancer Center (2018) Phase I/II bevacizumab versus bevacizumab plus TPI 287 for recurrent glioblastoma. https://clinicaltrials.gov/ct2/show/NCT01582152. Accessed 16 Feb 2022
Wetmore C, Boyett J, Li S, Lin T, Bendel A, Gajjar A, Orr BA (2015) Alisertib is active as single agent in recurrent atypical teratoid rhabdoid tumors in 4 children. Neuro Oncol 17(6):882–888. https://doi.org/10.1093/neuonc/nov017
Song A, Andrews DW, Werner-Wasik M, Kim L, Glass J, Bar-Ad V, Evans JJ, Farrell CJ, Judy KD, Daskalakis C, Zhan T, Shi W (2019) Phase I trial of alisertib with concurrent fractionated stereotactic re-irradiation for recurrent high grade gliomas. Radiother Oncol 132:135–141. https://doi.org/10.1016/j.radonc.2018.12.019
Takeda (Millennium Pharmaceuticals Inc) (2019) A phase I clinical and pharmacodynamic study of MLN8237, a novel Aurora A kinase inhibitor, in participants with advanced malignancies. ClinicalTrials.gov Identifier: NCT00651664. https://clinicaltrials.gov/ct2/show/NCT00651664. Accessed 16 Feb 2022
Falchook G, Coleman RL, Roszak A, Behbakht K, Matulonis U, Ray-Coquard I, Sawrycki P, Duska LR, Tew W, Ghamande S, Lesoin A, Schwartz PE, Buscema J, Fabbro M, Lortholary A, Goff B, Kurzrock R, Martin LP, Gray HJ, Fu S, Sheldon-Waniga E, Lin HM, Venkatakrishnan K, Zhou X, Leonard EJ, Schilder RJ (2019) Alisertib in combination with weekly paclitaxel in patients with advanced breast cancer or recurrent ovarian cancer: A randomized clinical trial. JAMA Oncol 5(1):183773
Sells TB, Chau R, Ecsedy JA, Gershman RE, Hoar K, Huck J, Janowick DA, Kadambi VJ, LeRoy PJ, Stirling M, Stroud SG, Vos TJ, Weatherhead GS, Wysong DR, Zhang M, Balani SK, Bolen JB, Manfredi MG, Claiborne CF (2015) MLN8054 and Alisertib (MLN8237): discovery of selective oral aurora a inhibitors. ACS Med Chem Lett 6(6):630–634. https://doi.org/10.1021/ml500409n
Du J, Yan L, Torres R, Gong X, Bian H, Marugan C, Boehnke K, Baquero C, Hui YH, Chapman SC, Yang Y, Zeng Y, Bogner SM, Foreman RT, Capen A, Donoho GP, Van Horn RD, Barnard DS, Dempsey JA, Beckmann RP, Marshall MS, Chio LC, Qian Y, Webster YW, Aggarwal A, Chu S, Bhattachar S, Stancato LF, Dowless MS, Iversen PW, Manro JR, Walgren JL, Halstead BW, Dieter MZ, Martinez R, Bhagwat SV, Kreklau EL, Lallena MJ, Ye XS, Patel BKR, Reinhard C, Plowman GD, Barda DA, Henry JR, Buchanan SG, Campbell RM (2019) Aurora A-selective inhibitor LY3295668 leads to dominant mitotic arrest, apoptosis in cancer cells, and shows potent preclinical antitumor efficacy. Mol Cancer Ther 18(12):2207–2219. https://doi.org/10.1158/1535-7163.MCT-18-0529
Mehta S, Tovmasyan A, Tien AC, Holter M, Hopkins B, Margaryan T, Chang YW, Himes S, Elliott M, Melendez EL, Pennington-Krygier C, White C, Molloy J, Liu M, Knight W, Sanai N (2021) Tumor pharmacokinetics, pharmacodynamics and radiation sensitization in patient-derived xenograft models of glioblastoma treated with the aurora kinase a inhibitor ly3295668. Neuro Oncol 23:165–165
Acknowledgements
We thank the Brown Cancer Animal Imaging Core Facility for performing magnetic resonance imaging. This work was supported by the National Institute of Health (NIH) grant R01-NS081125 (NLL).
Author information
Authors and Affiliations
Contributions
NLL BJW, JC, CTZ and MS contributed to the study design. Study conception and supervision was performed by NLL. Material preparation, data collection and analysis were performed by MS, BJW, CTZ, LT, MNGAl-K, AK, AJH, MJW, LMS, JC, and NLL. The first draft of the manuscript was written by MS and CTZ. All the authors commented on previous versions of the manuscript. All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
None of the authors has conflicting interest in the publication of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
280_2023_4503_MOESM1_ESM.eps
Supplementary file1 Fig. S1 GB9 and GB30 cell line growth comparison. Their proliferation rates (slopes) are significantly different (*p=0.0337), simple linear regression analysis (EPS 1518 KB)
280_2023_4503_MOESM2_ESM.pdf
Supplementary file2 Fig. S2 Western blotting of Bcl-2 family proteins in synchronized U87 cells. U87 cells were synchronized with double thymidine block, treated with 30 nM alisertib, 700 pM TPI 287, or both. Cell lysates were collected at 0, 24-, 48-, 72-, and 120 hr a. Western blotting of Bcl-2 family members. b. Bcl-2 c. Mcl-1 d. Bak and e. Bim protein level quantification (PDF 885 KB). Circles, squares, triangles and upside-down triangles represent each biological replicate for vehicle, alisertib, TPI 287, and alisertib + TPI 287 treatments, respectively. Representative blots, n = 3, two-way ANOVA, p values
280_2023_4503_MOESM3_ESM.eps
Supplementary file3 Fig. S3 Western blotting for a. Bim and b. Bak knockdown confirmation in GB9, U87 and GB30 cells (EPS 1872 KB)
Supplementary file4 Supplementary Video 1. Representative 72 hr time-lapse video of GB9 cells embedded in 3D Matrigel treated with vehicle (AVI 28731 KB)
Supplementary file5 Supplementary Video 2. Representative 72 hr time-lapse video of GB9 cells embedded in 3D Matrigel treated with 30 nM alisertib (AVI 22104 KB)
Supplementary file6 Supplementary Video 3. Representative 72 hr time-lapse video of GB9 cells embedded in 3D Matrigel treated with 1 nM TPI 287 (AVI 19041 KB)
Supplementary file7 Supplementary Video 4. Representative 72 hr time-lapse video of GB9 cells embedded in 3D Matrigel treated with 30 nM alisertib + 1 nM TPI 287 (AVI 24110 KB)
Supplementary file8 Supplementary Video 5. Representative 72 hr time-lapse video of GB30 cells embedded in 3D Matrigel treated with vehicle (AVI 29535 KB)
Supplementary file9 Supplementary Video 6. Representative 72 hr time-lapse video of GB30 cells embedded in 3D Matrigel treated with 30 nM alisertib (AVI 20976 KB)
Supplementary file10 Supplementary Video 7. Representative 72 hr time-lapse video of GB30 cells embedded in 3D Matrigel treated with 1 nM TPI 287 (AVI 23741 KB)
Supplementary file11 Supplementary Video 8. Representative 72 hr time-lapse video of GB30 cells embedded in 3D Matrigel treated with 30 nM alisertib + 1 nM TPI 287 (AVI 20271 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sak, M., Williams, B.J., Zumbar, C.T. et al. The CNS-penetrating taxane drug TPI 287 potentiates antiglioma activity of the AURKA inhibitor alisertib in vivo. Cancer Chemother Pharmacol 91, 191–201 (2023). https://doi.org/10.1007/s00280-023-04503-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-023-04503-0