Abstract
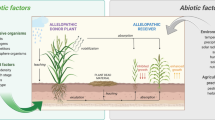
Many invasive alien species (IAS) produce secondary metabolites that affect how other plants function (allelopathic compounds) and can drive other species invasion, as proposed by the invasional meltdown hypothesis. Acacia melanoxylon and Eucalyptus globulus are two of such species. In this study, we analyzed the germination response of seven IAS (Acacia dealbata, Acacia mearnsii, Acacia melanoxylon, Acacia longifolia, Eucalyptus globulus, Paraserianthes lophantha, Phytolacca americana) and a native biotest species (Lactuca sativa) to the application of two different aqueous extracts at two different concentrations of donor species A. melanoxylon and E. globulus. Extract compounds were identified by UHPLC-ESI-QTOF-MS. Eucalyptus aqueous extracts significantly reduced germination in three species (A. dealbata, E. globulus, P. americana). The germination of all the species tested was reduced with acacia aqueous extracts. Our results support the postulates of the Biochemical Recognition Hypothesis in that seeds gauge establishment potential based on phytochemical release of other plants. Furthermore, A. melanoxylon and E. globulus lowered their own germination, suggesting that these species exhibit intraspecific biochemical recognition. We also found support for the Novel Weapons Hypothesis in the case of L. sativa as a native species. Our research shows that phytochemicals are a component of plant-plant interactions, including the invasion process.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Invasive alien species (IAS) are a threat to global biodiversity, because they outcompete native species, change the physico-chemical characteristics of soils (e.g., modification of the soil microbial activity—Qu et al. 2021; allelochemical release that reduces native plant establishment—Zhang et al. 2021), alter the rate of nutrient cycling (Vilà et al. 2011) and modify ecosystem fire regimes (D’Antonio and Vitousek 1992; Gaertner et al. 2014). Plant invaders reduce the richness and abundance of native species (Pyšek et al. 2012; Wohlgemuth et al. 2022) by modulating seed germination, inhibit seedling establishment and growth (Hussain et al. 2011a), modification of plant-pollinator interactions, and pose a significant impact at species, ecosystem and community levels and alter ecosystem services (Vilà et al. 2011). Additionally, IAS cause economic loss in forestry and agriculture (Pimentel et al. 2005) and are accelerated by anthropogenic perturbations (Young and Larson 2011). Coastal areas with temperate climates are a primary pathway for the introduction of IAS to Europe and the level of invasion in these areas is usually high (Chytrý et al. 2009) due to its favorable climate for plant establishment and growth (Stohlgren et al. 2003, 2006). The northwestern Iberian Peninsula constitutes a pathway of entry of IAS to Europe, and exhibits some early stages of IAS invasion (Capdevila-Argüelles et al. 2012; GISD 2022). This coastal region also has the highest forest fire activity in Europe (San-Miguel-Ayanz et al. 2013) and is one of the most affected regions by fire in the world (Archibald et al. 2013). Some of the IAS are tree species that cause major ecosystem problems, including the propagation of forest fires and making areas more fire prone (Brooks et al. 2004; Underwood et al. 2019; Aslan and Dickson 2020). In the northwest of the Iberian Peninsula several species stand out, including widely distributed species of Acacia and Eucalyptus genera (Sanz-Elorza et al. 2004), as well as others in early stages of invasion, such as the genera Paraserianthes (Dana et al. 2004) and Phytolacca (Valdés et al. 2011).
The invasional meltdown hypothesis (Simberloff and von Holle 1999) states that the establishment of one IAS facilitates invasion of other IAS (Kumar Rai and Singh 2020). Testing this hypothesis is the first step to know the repercussion of the initial invasions on subsequent ones and will be useful for exposing possible key species in the invasion process. Acacia melanoxylon R. Br. (Australian Blackwood) is one of the most damaging species for agriculture (Souto et al. 1994; Hussain et al. 2020) and Eucalyptus globulus Labill. (blue gum) for forestry ecosystems (Arán et al. 2013; Calviño-Cancela and Rubido-Bará 2013). Testing the invasional meltdown hypothesis for these IAS will allow us to discover if their presence helps the establishment of other IAS, namely other acacias, Paraserianthes lophantha (Willd.) I.C.Nielsen and Phytolacca americana L. This hypothesis postulates an adverse IAS diversity synergism on native species. Invasive species can also drive their own regeneration or produce negative feedback that limit it.
Seeds can recognize phytochemicals of conspecific seeds and establishing plants (biochemical recognition hypothesis, BRH; sensu Renne et al. 2004, 2014). Based on their dose-dependent assessment, they may decide to accelerate (e.g., avoid priority effects or enjoy conspecific facilitation; Dyer 2004; Orrock and Christopher 2010; Yamowo and Mukai 2017; Yannelli et al. 2020; Ohsaki et al. 2020) or delay germination (e.g., avoid competition-induced post-emergence mortality; Renne et al. 2014; Houseman and Mahoney 2015) to maximize establishment potential. In either case, this competition avoidance mechanism is predicted to maximize establishment potential (Renne et al. 2014) and thus, invasion success. This process also applies to heterospecific recognition (Tielbörger and Prasse 2009; Yannelli et al. 2020; Fenesi et al. 2020). Some invasive plants may succeed because they bring novel phytotoxins to natural plant communities, as proposed by Callaway and Aschehoug (2000) in the novel weapons hypothesis (NWH). This would be a non-mutually exclusive mechanism to the BRH, for their own population’s regeneration. Following this statement, native species would be more vulnerable to allelochemicals that they had never encountered. This is a chemically mediated mechanism that has been demonstrated in several studies (Callaway and Ridenour 2004; Becerra et al. 2018; Puig et al. 2018), and affects other species germination or seedling growth. This knowledge will be very useful for policymaking on control and management of these invasive alien species due to its wide distribution around the world (GISD 2022) since their interactions and synergies could also take place in other locations.
Many introduced species of Acacia and Eucalyptus produce allelopathic compounds, which reduce germination of some species (Souto et al. 2001; Hussain et al. 2011b; Lorenzo et al. 2012). Allelopathy is the ecological process in which biotic interference occurs through bioactive molecules (Singh et al. 1999). Allelochemicals (i.e., secondary metabolites, mostly phenolics and terpenoids) usually reduce native species and facilitate for IAS colonization in new habitats (Novoa et al. 2012; Kalisz et al. 2021; Zhang et al. 2021). The identification of phenolics, flavonoids and terpenoids can be difficult because they contain several structures but HPLS-MS is a useful tool to identify natural compounds in vegetal extracts (Quirantes-Piné et al. 2013; Jia et al. 2016). Allelochemicals could drive the invasion success of IAS, and it is important to know which compounds or doses affect their own germination and that of other species. Since germination is the most critical stage in the life cycle of many plants (Reyes et al. 1997) it is necessary to know how IAS seeds respond to their own phytochemicals and how the allelochemicals affect other IAS germination in already colonized areas.
In this study, we addressed the following objectives: (i) to determine the effect of different doses of allelopathic compounds from aqueous extracts of A. melanoxylon phyllodes and E. globulus leaves on their own germination and on other IAS germination, and (ii) to identify the possible compounds responsible for germination modification.
Materials and methods
Study species
We studied seven invasive alien species present in the northwest of the Iberian Peninsula and found in many other areas of the world: Acacia dealbata Link, Acacia mearnsii De Wild., Acacia melanoxylon R.Br., Acacia longifolia (Andrews) Willd., Eucalyptus globulus Labill., Paraserianthes lophantha (Willd.) I.C.Nielsen and Phytolacca americana L. The seeds of test species were collected in naturalized IAS populations in Galicia, Spain (northwestern Iberian Peninsula, Table S1). Additionally, we used Lactuca sativa L (cv. “Batavia”) commercial seeds as a biotest species due to their fast germination and common use in allelopathic bioassays; seeds were purchased from Wamestrada S.L.L. (A Estrada, Spain).
Allelochemicals water extraction
Acacia melanoxylon and E. globulus were chosen as donor species of allelopathic compounds, due to their invasive character, their wide distribution and the density of their populations in the study area. Acacia melanoxylon is one of the IAS that has greatly increased in abundance and distribution in recent years (Martínez-Fernández et al. 2012); E. globulus is one of the most abundant species in the northwestern Iberian Peninsula, occupying 20% of the forested areas according to the IFN4 (IFN4 2011). Four aqueous extracts constituted the test treatments of IAS germination: two extracts at different concentrations from each of the two donor species. For the acacia extracts, we used A. melanoxylon abscised dry phyllodes and for the eucalyptus extracts we used E. globulus abscised dry leaves. Phyllodes and leaves were collected in naturalized adult populations in the Monte Pedroso area (Galicia, Spain). After collection, phyllodes and leaves were slashed in 2 cm2 pieces (~ 1 × 2 cm). Extraction was performed in beakers covered with plastic film by soaking slashed phyllodes or leaves in distilled water at 200 g/L (hereafter, 200 g-acacia and 200 g-eucalyptus) and 100 g/L (100 g-acacia and 100 g-eucalyptus) for 72 h at room temperature. Then, leaves and phyllodes were separated from water by filtration and discarded, leading to the aqueous extracts. Following a similar methodology to Teerarak et al. (2010) and Nurjanah et al. (2020), the chosen values corresponded to the phyllodes or leaves maximum amount that is possible to soak in a known amount of water (200 g of phyllodes or leaves slashed pieces in 1 L of distilled water) and then reducing this amount to a half (100 g in 1 L).
Germination bioassays
For each of the seven species studied, in addition to four aqueous extract treatments, we used distilled water as a control to simulate natural conditions (control treatment). Hard-coated seeds were mechanically scarified before the beginning of the test to simulate germination. Scarification was performed with a scalpel, transversally cutting off a small part of the seed’s distal end. For each treatment, five replicates of twenty-five seeds each were made. Each replicate was placed on a 9 cm diameter Petri dish, using two cellulose filter papers as substrate.
At the beginning of the test, 4 ml of the corresponding aqueous extract or distilled water was added to each replicate (Salgado et al. 2017). Subsequently, seed germination was checked every other day during a month. On those days, more water was added to keep the seeds moist, and seeds that had germinated (visible radicle) were removed from the Petri dish. Seed incubation was performed in a germination chamber (Climas AGP890) where the thermo-photoperiod was 16 h of light at 24 °C and 8 h of darkness at 16 °C, simulating favorable conditions for germination in the northwest of the Iberian Peninsula (Cruz et al. 2019; Riveiro et al. 2020).
Compound identification
To identify the A. melanoxylon and E. globulus chemical constituents present in the aqueous extracts, we conducted an ultrahigh-performance liquid chromatography coupled with quadrupole time-of-flight tandem mass spectrometry (UHPLC-ESI-QTOF-MS), a tool for characterizing complex natural products (Li et al., 2017). The compounds were identified by comparing their retention times and MS/MS spectra provided by QTOF–MS with those of authentic standards whenever available. The remaining compounds were identified by interpreting their MS and MS/MS spectra obtained by QTOF–MS combined with the data provided in the literature. The score is a measure of identification confidence, based on the parameters of exact mass, retention time, mSigma and qualifier ions. Five score levels were obtained based on this: high (++++/+++), medium (++), tentative (+) and null (-); null level means that the qualifier ion was not found.
Data analysis
The data obtained were used to calculate the average germination percentage, the germination over time and the germination speed as T50 (which measures germination speed as the time required by seeds to reach 50% of final germination). T50 was calculated according to Cruz et al. (2022).
General linear models (GLMs) at a significance level of 0.05, with binomial error distribution, were carried out to test the effects of acacia and eucalyptus extracts on germination percentage and T50 (see supplementary material, Table S2). Independent analyses for each target species were carried out in a factorial analysis with the fixed factors extract source and concentration of the extract. Interaction between extract source and concentration of the extract was tested but it was not significant in any of the analyses, so we did not include it in the model to have a better model fit. We tested whether there were significant differences in extract source and concentration of the extract through ANOVA using the package car (Fox and Weisberg 2019); followed by a post-hoc HSD Tukey test using the package agricolae (de Mendiburu and Yassen 2020). GLMs for target species E. globulus and L. sativa regarding the response variable T50 did not include the predictor variable concentration of the extract for acacia extracts owing to the lack of data (there was no germination under these treatments). All the analyses were performed using R Software (R Core Team 2023).
Results
Germination percentage
Final germination varied by species and treatment (Table 1). Germination of all test species were significantly reduced by at least one treatment, and no treatment promoted germination. The germination percentage of A. dealbata and P. lophantha decreased in all treatments with respect to control and was especially low with 200 g-acacia treatment. Germination of A. dealbata decreased by 33% with 100 g-eucalyptus, 200 g-eucalyptus and 100 g-acacia and 75% with 200 g-acacia. Paraserianthes lophantha germination decreased 40% from control treatment with 200 g-acacia treatment (p < 0.013) and none of the other treatments modified its germination percentage. Germination of A. mearnsii decreased 36% with 200 g-acacia treatment (p < 0.04) with respect to control. Acacia longifolia and A. melanoxylon seed germination percentage was significantly reduced with both 100 g-acacia (61%, 87% respectively) and 200 g-acacia (71%, 68% respectively) treatments. Other treatments did not affect germination.
In E. globulus, the 200 g-acacia treatment inhibited germination. 200 g-eucalyptus and 100 g-acacia reduced it by 70% and 53% with respect to control. Almost all treatments applied to P. americana significantly reduced germination (p < 0.001); 200 g-acacia reduced it by 43%, and 100 g-acacia and 200 g-eucalyptus produced smaller reductions (24%, 19% respectively). 100 g-eucalyptus did not affect germination of these two species. Biotest species L. sativa showed a marked decrease in germination percentage with 200 g-acacia (p < 0.001) from 89.6% in control treatment to 0%; other treatments did not affect its germination.
Acacia melanoxylon reduced target species germination with low (100 g-acacia) and high (200 g-acacia) concentration extracts, including its extracts to its own seeds. Regarding E. globulus, low concentration extract (100 g-eucalyptus) did not modify its own germination, but high concentrations (200 g-eucalyptus) reduced it. This effect was similar to that produced by E. globulus extracts in the other species studied, in which high concentration extract reduced germination more than low concentration extracts.
The most affected species by the applied treatments were A. dealbata, E. globulus and P. americana, whose germination was significantly reduced by 3 of the 4 treatments tested. In contrast, relative to the control, extracts generally did not affect the germination of A. mearnsii, P. lophantha and L. sativa, since their germination was reduced by only 1 out of 4 treatments. Eucalyptus aqueous extracts inhibited germination in 4 out of the 16 tests, and acacia aqueous extracts were inhibitory in 13 out of the 16 tests performed. Overall, the treatment which had the strongest effect was 200 g-acacia, which significantly reduced the germination percentage of seeds in all the species studied. 100 g-eucalyptus had the weakest effect, only modifying the germination of 1 out of 8 species.
T50 and temporal distribution
For most of the species studied, T50 increased slightly with some of the treatments, but the statistical analyses were not significant. The treatments applied only lead to a significant delay in the T50 for A. dealbata and L. sativa (p < 0.004 and p < 0.001, respectively). The control germination of A. dealbata started on day 3 (Fig. 1) and ended on day 18, with a T50 of 3 days. All treatments significantly increased the T50 of this species (Table 1), reaching 5.0 to 6.2 days. Treated seeds had a delayed germination start on day 5. Control T50 of L. sativa biotest species was 2.0 days, which significantly increased to 5.0 days (p < 0.05) for the seeds treated with 100 g-eucalyptus, 200 g-eucalyptus and 100 g-acacia. The control germination of L. sativa started on day 3, while the seeds’ germination treated with 100 g-acacia started on day 5, and with 100 g-eucalyptus and 200 g-eucalyptus, germination started on day 9.
Acacia longifolia had the longest T50 at 8 days, while all the other species reached T50 at 5 days. In A. mearnsii control germination started on day 3 (Fig. 1) and reached a T50 of 5.0 days. The control germination of A. melanoxylon, E. globulus and P. americana started on day 5, and reached T50 on day 5 (Table 1). Treated seeds of A. melanoxylon started germination on day 9, delaying it with respect to control. Eucalyptus globulus seeds treated with 100 g-acacia started germination on the same day as control (Fig. 1) while seeds treated with 100 g-eucalyptus and 200 g-eucalyptus delayed their germination. Neither A. melanoxylon nor E. globulus modified their own T50.
Germination of A. longifolia started on day 5 in control and treated seeds, with a T50 of 8.2 days and none of the treatments modified it (Table 1). Control seeds of P. lophantha and P. americana began their germination on day 3 while treated seeds started germination on day 5 (Fig. 1). The T50 of P. lophantha was very consistent, with no variation between control and treatments, being 5.0 days on average (Table 1).
To sum up, the T50 of most species remained unchanged after the application of treatments compared to control, except for A. dealbata and L. sativa in which case it was delayed (Table 1). Eucalyptus aqueous extracts delayed T50 in 4 out of 16 treatments, and acacia aqueous extracts delayed it in 3 out of 14 (Table 1). The treatments also delayed the start of germination, ranging from 2 to 6 days in all species except A. longifolia, whose treated seeds started germinating the same day as the control seeds (Fig. 1).
Identification and chemical analysis of compounds in aqueous extracts
The eucalyptus extracts contained 47 compounds, while acacia extracts had 20 compounds (Table S3, supplementary material). The maximum confidence level obtained in acacia extracts was medium (++), in 8 compounds and none of the compounds reached the highest level. From these compounds, the most abundant were trihydroxyoctadecenoic acid (17.5%), tyrosol glucoside (3.6%), gentistic acid (2.5%) and scopoletin (2.0%). Eleven compounds of eucalyptus extract obtained medium confidence level (++) and 4 compounds obtained a high level (++++). The high confidence level compounds were gallic acid, epicatechin, ferulic acid and ellagic acid, which represents 39.4% of the total eucalyptus sample analyzed. Thus, gallic acid and ellagic acid were the two main compounds in Eucalyptus extracts, and two of the most certain in the sample.
Eucalyptus and Acacia extracts shared 7 out of 16 high confidence level compounds. However, gallic acid and ellagic acid, the two most abundant compounds in eucalyptus extracts, were not present in the acacia extracts. Only 1 of the 16 high confidence compounds, tyrosol glucoside, was present in acacia extracts but not in eucalyptus extracts.
Discussion
Germination and ecological implications
Acacia and eucalyptus phytochemical compounds affected germination of the eight study species, and aqueous extract dose and origin were fundamental to this. Extracts of A. melanoxylon lowered germination in all species treated with 200 g-acacia, E. globulus extracts decreased germination in A. dealbata, E. globulus and P. americana, and none of the extracts fostered germination in any species. The germination reduction in all the studied species by A. melanoxylon extracts, at least at the highest concentration (200 g-acacia), matches the results of Hussain et al. (2011), who concluded that Acacia allelopathy effects depend on extract concentration. Of the five species whose germination was inhibited with the two concentrations tested, the inhibition effect increased with incrementing extract concentration only in A. dealbata and P. americana. The lack of germination promotion in any target species, does not support the invasional meltdown hypothesis (Simberloff and von Holle 1999). For example, A. melanoxylon and E. globulus did not facilitate the invasion of A. dealbata, A. mearnsii, P. lophantha and P. americana through allelopathy compounds in areas already colonized by A. melanoxylon or E. globulus. This is consistent with the fact that the majority of interactions between invaders are either neutral or negative (81% of interactions) and it is very rare the facilitation among them (Kuebbing and Nuñez 2015).
Regarding their native range, all target acacias are sympatric—as well as congeneric—so it is possible they have developed species-specific or phylogenetic-specific BR (Renne et al. 2014). The lowered acacia germination with A. melanoxylon extracts do not support the NWH, because the phytotoxins would not be new to the target acacias and it is unlikely that sharing habitat both in their native range and in their invaded range to not have developed some sort of mechanism to avoid the phytotoxic effects of sympatric, closely-related species. Importantly, A. melanoxylon extracts (100 g-acacia and 200 g-acacia) reduced germination of A. melanoxylon seeds, and 200 g-eucalyptus reduced E. globulus germination. This was found in other Fabaceae species (Khan and Shaukat 2006) treated with its own fruit extracts and in other woody species (Bran et al. 1990; Romane et al. 1992; Houseman and Mahoney 2015). The fact that the germination reduction is stronger on donor species than on the other IAS clearly supports the BRH. Donor species would be able to recognize high population density through phytochemicals released into the environment (intraspecific-BR) and react by limiting their germination to avoid strong intraspecific competition in the future (Renne et al. 2014; Hierro and Callaway 2021). This effect of suppression of its own germination is not detected in natural populations, probably due to the potential seed bank that those species presents; in A. melanoxylon the soil seed bank is large and persistent (Arán et al. 2017)d globulus has an aerial seed bank in which seeds can remain viable during years (Reyes and Casal 1998). Specifically, A. melanoxylon, in which we found the strongest germination reduction, tends to form dense monospecific stands which allow them to be permanently in contact with their conspecific phytochemicals, favoring BR development (Renne et al. 2014). The regulation of populational growth via BR would be ideal conditions for seed germination, with adult individuals releasing phytochemicals that postpone germination until the environment is conducive for establishment. This behavior would benefit adult plants of A. melanoxylon and E. globulus, as described by Aguilera et al. (2017) for A. dealbata in Chile.
Germination of biotest L. sativa was reduced with acacia extracts and delayed by all applied treatments. Similar results were obtained by Souto et al. 2001, who found reduced germination of L. sativa seeds from A. melanoxylon stands. Also, Hussain et al. (2011a), 2020) reported inhibition of germination of L. sativa seeds treated with flower and phyllodes extracts of A. melanoxylon; they also detected native species inhibition in Atlantic forests, such as Dactylis glomerata L., Lolium perenne L. and Rumex acetosella L. Meanwhile, E. globulus extracts did not affect L. sativa germination, neither in our study nor in Souto et al. (2001). Given that L. sativa is a native species, our results regarding acacia extracts support the NWH (Callaway and Aschehoug 2000). Nelson et al. (2021) did not find inhibitory effects of E. globulus on the germination of understory Californian native species either. By contrast Morsi and Abdelmigid (2016) found inhibitory effects on Hordeum vulgare L. germination, a native species of Saudi Arabian forests, using a high concentration of E. globulus leaf extracts. Additionally, despite its common use as biotest species in allelopathy studies, we are aware of the limitations of using L. sativa as a native species, due to its commercial use and its artificial selection for agriculture. For these reasons, it is necessary to perform more tests on a wider range of native species to extract solid conclusions about the NWH for the two donor species.
Different extracts and doses barely modified speed and temporal distribution of germination. In A. dealbata, treated seeds took longer to reach T50 when compared to control treatment. This also occurred in L. sativa, whose treated seeds also started germination later than control treatment. Other authors found that reduced germination is not linked to changes in T50 (Cruz et al. 2020). Start of germination was also delayed in some target species between 2 and 6 days; a delay in germination onset could be due to BR, since we only applied the aqueous extracts at the start of the experiment, and subsequently applied distilled water. It is possible that phytochemical concentration in the seed environment was reduced, leading to more conducive conditions for germination. Comparing the eight studied species, the most sensitive ones were A. dealbata, E. globulus and P. americana, whose germination was lowered by three out of four tested treatments. Furthermore, the only species whose germination was totally inhibited were L. sativa and E. globulus, which were treated with 200 g-acacia. These two species were the only ones studied that did not present hard-coated seeds and had the smallest seed size. Nevertheless, all hard-coated seeds used in this study were scarified, so we cannot attribute this effect to the protective coat. In normal conditions, with the hard coat unaltered, these species take 1 to 3 months to germinate and their germination is low (Arán et al. 2017; Cruz et al. 2017; García-Duro et al. 2019; Riveiro et al. 2020).
Aqueous extract composition
Some of the main compounds found in the A. melanoxylon chemical analysis were reported in previous allelopathy studies. p-Hydroxybenzoic acid (Reigosa et al. 1999; Hussain et al. 2011b) was even reported to cause germination suppression and radicle growth inhibition at different concentrations in several species. In our study, A. melanoxylon extracts did not contain gallic acid, ferulic acid (Souto et al. 1994; Hussain et al. 2011b) and ellagic acid (Souto et al. 1994) despite being tested for them. Instead, we found that the most abundant compounds were trihydroxyoctadecenoic acid and tyrosol glucoside, which were not previously reported in A. melanoxylon extracts. In E. globulus extracts, the principal compounds gallic acid, epicatechin, ferulic acid and ellagic acid were found in other studies (Souto et al. 1994; Reigosa et al. 2000; Hussain et al. 2011b) and in other species of the Eucalyptus genus (Suresh and Vinaya Rai 1987; Li et al. 2010). Gallic acid and chlorogenic acid were also found by Puig et al. (2018) who concluded that the inhibitory effects observed could be attributed a priori to the phytotoxins present in the plant’s aqueous extracts. Some of the compounds identified in our study also cause growth suppression in seedlings (Puig et al. 2018); Hussain et al. (2010) found a reduction on shoot, leaf and root length and a drop in fresh biomass of L. sativa using ferulic acid. This effect on the seedling growth would support the NWH if we use L. sativa to depict native species that have never been exposed to IAS allelochemicals of tested donor species, and consequently would experience negative effects after the exposure.
It would be interesting to clarify if the compounds found in our aqueous extracts cause inhibitory effects on germination when isolated from the mixtures. Other authors (Chon et al. 2003; Reigosa and Pazos-Malvido 2007) tested the efficacy of these compounds as germination suppressors, and they concluded that the compound’s mixtures were more phytotoxic than individual compounds. As we did not find phytotoxic effects on IAS but biochemical recognition among them, we wonder if the BR would be stronger in the compound mixture than in the isolated compounds, or in contrast, whether there would be some compounds responsible for the BR. Furthermore, the allelochemical effect could be a temporary germination inhibition, reversible when the allopathic compound concentration decreases, and the environment is more conducive for establishment. Lastly, given that seven of sixteen compounds are shared between acacia and eucalyptus test species (Table 2), it is possible that seeds use these phytogeneric cues to indicate a competitive environment (Renne et al. 2014), regardless of whether an established acacia or eucalyptus individual is in close proximity.
Conclusion
From the seven studied IAS, only three were affected by adding eucalyptus aqueous extracts. In contrast, all of them were affected by acacia aqueous extracts. Regarding the treatments, eucalyptus aqueous extracts lowered germination in 4 out of 16 tests, and acacia aqueous extracts were inhibitory in 13 out of 16 tests performed. In all cases, the treatment that most reduced germination was 200 g-acacia (acacia aqueous extract at higher concentration). We also detected germination reduction in the two donor species treated with their own phytochemicals, suggesting intraspecific BR, and found support for the NWH depicting L. sativa as a native species. None of the donor species promoted germination in the tested species, discarding the meltdown hypothesis for A. melanoxylon and E. globulus aqueous extracts. In conclusion, our findings show that phytochemicals mediate plant-plant interactions by altering germination schedules, which likely scale up to population and community regulation.
Data Availability
All data are available under request at Zenodo under the DOI: https://doi.org/10.5281/zenodo.5910035.
Reference
Aguilera N, Guedes LM, Becerra J, González L (2017) Is autotoxicity responsible for inhibition growth of new conspecific seedlings under the canopy of the invasive Acacia dealbata link? Gayana Botánica 74:1–14. https://doi.org/10.4067/S0717-66432017005000101
Arán D, García-Duro J, Reyes O, Casal M (2013) Fire and invasive species: modifications in the germination potential of Acacia melanoxylon, Conyza canadensis and Eucalyptus globulus. For Ecol Manage 302:713. https://doi.org/10.1016/j.foreco.2013.02.030
Arán D, García-Duro J, Cruz Ó et al (2017) Understanding biological characteristics of Acacia melanoxylon in relation to fire to implement control measurements. Ann for Sci 74:61. https://doi.org/10.1007/s13595-017-0661-y
Archibald S, Lehmann CER, Gómez-Dans JL, Bradstock RA (2013) Defining pyromes and global syndromes of fire regimes. Proc Natl Acad Sci 110:6442–6447. https://doi.org/10.1073/pnas.1211466110
Aslan CE, Dickson BG (2020) Non-native plants exert strong but under-studied influence on fire dynamics. NeoBiota 61:47–64. https://doi.org/10.3897/NEOBIOTA.61.51141
Becerra PI, Catford JA, Inderjit et al (2018) Inhibitory effects of Eucalyptus globulus on understorey plant growth and species richness are greater in non-native regions. Glob Ecol Biogeogr 27:68–76. https://doi.org/10.1111/GEB.12676
Bran D, Lobréaux O, Maistre M et al (1990) Germination of Quercus ilex and Q. pubescens in a Q. ilex coppice - long-term consequences. Vegetatio 87:45–50. https://doi.org/10.1007/BF00045654
Brooks ML, D’Antonio CM, Richardson DM et al (2004) Effects of invasive alien plants on Fire Regimes | BioScience | Oxford Academic. Bioscience 54:677–688. https://doi.org/10.1641/0006-3568(2004)054[0677:EOIAPO]2.0.CO;2
Callaway RM, Aschehoug ET (2000) Invasive plants versus their new and old neighbors: a mechanism for exotic invasion. Sci (80-) 290:521–523. https://doi.org/10.1126/SCIENCE.290.5491.521/ASSET/C45238A7-1E34-42FC-ADAF-9A1315822366/ASSETS/GRAPHIC/SE4008916004.JPEG
Callaway RM, Ridenour WM (2004) Novel weapons: invasive success and the evolution of increased competitive ability - callaway – 2004 - frontiers in Ecology and the Environment - Wiley Online Library. Front Ecol Environ 2:436–443. https://doi.org/10.1890/1540-9295(2004)002[0436:NWISAT]2.0.CO;2
Calviño-Cancela M, Rubido-Bará M (2013) Invasive potential of Eucalyptus globulus: seed dispersal, seedling recruitment and survival in habitats surrounding plantations. For Ecol Manage 305:129–137. https://doi.org/10.1016/J.FORECO.2013.05.037
Capdevila-Argüelles L, Zilletti B, Suárez Álvarez V (2012) Plan Estratéxico Galego de xestión das Especies Exóticas Invasoras e para o desenvolvemento dun sistema estandarizado de. Análise de Risco para as especies exóticas en Galicia
Chon S-U, Kim Y-M, Lee J-C (2003) Herbicidal potential and quantification of causative allelochemicals from several Compositae weeds. Weed Res 43:444–450. https://doi.org/10.1046/j.0043-1737.2003.00361.x
Chytrý M, Pyšek P, Wild J et al (2009) European map of alien plant invasions based on the quantitative assessment across habitats. Divers Distrib 15:98–107. https://doi.org/10.1111/j.1472-4642.2008.00515.x
R Core Team (2023) R: A language and environment for statistical computing
Cruz Ó, García-Duro J, Casal M, Reyes O (2017) Can the mother plant age of Acacia melanoxylon (Leguminosae) modulate the germinative response to fire? Aust J Bot 65:593. https://doi.org/10.1071/BT17083
Cruz Ó, García-Duro J, Casal M, Reyes O (2019) Role of serotiny on Pinus pinaster Aiton germination and its relation to mother plant age and fire severity. iForest 12:491–497. https://doi.org/10.3832/ifor2968-012
Cruz Ó, García-Duro J, Riveiro SF et al (2020) Fire severity drives the natural regeneration of Cytisus scoparius L. (link) and Salix atrocinerea brot. Communities and the germinative behaviour of these species. Forests 11:124. https://doi.org/10.3390/f11020124
Cruz Ó, Riveiro SF, Casal M, Reyes O (2022) Effect of fire factors (smoke, ash, charcoal and heat) on seeds of plant species. MethodsX 9:101679. https://doi.org/10.1016/J.MEX.2022.101679
D’Antonio CM, Vitousek PM (1992) Biological Invasions by exotic grasses, the Grass/Fire cycle, and global change. Annu Rev Ecol Syst 23:63–87. https://doi.org/10.1146/annurev.es.23.110192.000431
Dana ED, Sobrino E, Sanz-Elorza M (2004) Plantas invasoras en España: un nuevo problema en las estrategias de conservación. In: Bañares Á, Blanca G, Gümes J et al (eds) Atlas y libro rojo de la flora vascular amenazada. Dirección General de Conservación de la Naturaleza, Madrid, pp 1010–1029
de Mendiburu F, Yassen M (2020) agricolae: Statistical Procedures for Agricultural Research
Dyer AR (2004) Maternal and sibling factors induce dormancy in dimorphic seed pairs of Aegilops triuncialis. Plant Ecol 172:211–218. https://doi.org/10.1023/B:VEGE.0000026339.61069.33/METRICS
Fenesi A, Kelemen K, Sándor D et al (2020) Influential neighbours: seeds of dominant species affect the germination of common grassland species. J Veg Sci 31:1028–1038. https://doi.org/10.1111/JVS.12892
Fox J, Weisberg S (2019) An R companion to Applied Regression, 3rd edn. Sage, Thousand Oaks (CA)
Gaertner M, Biggs R, Te Beest M et al (2014) Invasive plants as drivers of regime shifts: identifying high-priority invaders that alter feedback relationships. Divers Distrib 20:733–744. https://doi.org/10.1111/ddi.12182
García-Duro J, Cruz Ó, Casal M, Reyes O (2019) Fire as driver of the expansion of Paraserianthes lophantha (Willd.) I. C. Nielsen in SW Europe. Biol Invasions 21:1427–1438. https://doi.org/10.1007/s10530-018-01910-w
GISD (2022) Global Invasive Species Database (GISD). https://www.cabi.org/isc/abstract/20067200496. Accessed 14 Dec 2020
Herrero-Borgoñón J (2007) Dos Mimosoideas (Leguminosae) nuevas para la flora castellonense. Flora Montiberica 28:26–28
Hierro JL, Callaway RM (2021) The ecological importance of Allelopathy. Annu Rev 52:25–45. https://doi.org/10.1146/ANNUREV-ECOLSYS-051120-030619
Houseman GR, Mahoney AK (2015) Intraspecific seed interactions alter seedling emergence of Lespedeza cuneata under field conditions. Popul Ecol 57:539–544. https://doi.org/10.1007/S10144-015-0495-0
Hussain MI, González L, Reigosa MJ (2010) Phytotoxic effects of allelochemicals and herbicides on photosynthesis, growth and carbon isotope discrimination in Lactuca sativa. Allelophaty J 26:157–174
Hussain MI, González L, Reigosa MJ (2011a) Allelopathic potential of Acacia melanoxylon on the germination and root growth of native species. Weed Biol Manag 11:18–28. https://doi.org/10.1111/j.1445-6664.2011.00401.x
Hussain MI, González L, Souto XC, Reigosa MJ (2011b) Ecophysiological responses of three native herbs to phytotoxic potential of invasive Acacia melanoxylon R. Br. Agrofor Syst 83:149–166. https://doi.org/10.1007/s10457-011-9433-0
Hussain MI, El-Sheikh MA, Reigosa MJ (2020) Allelopathic potential of aqueous extract from acacia melanoxylon r. br. On lactuca sativa. Plants 9:1–13. https://doi.org/10.3390/plants9091228
IFN4 (2011) Cuarto Inventario Forestal Nacional, Galicia
Jia M, Li Y, Zhai X et al (2016) Qualitative analysis and quality evaluation of Cnidium monnieri using UHPLC-ESI-Q-TOF/MS. Chin Herb Med 8:323–330. https://doi.org/10.1016/s1674-6384(16)60058-8
Kalisz S, Kivlin SN, Bialic-Murphy L (2021) Allelopathy is pervasive in invasive plants. Biol Invasions 23:367–371. https://doi.org/10.1007/S10530-020-02383-6/FIGURES/1
Khan D, Shaukat S (2006) Phytotoxic effects of Prosopis juliflora Swartz. DC. Against some of its field associates and the cultivated species. Int J Biol Biotechnol 3:353–366
Kuebbing SE, Nuñez MA (2015) Negative, neutral, and positive interactions among nonnative plants: patterns, processes, and management implications. Glob Chang Biol 21:926–934. https://doi.org/10.1111/GCB.12711
Kumar Rai P, Singh JS (2020) Invasive alien plant species: their impact on environment, ecosystem services and human health. Ecol Indic 111:106020. https://doi.org/10.1016/j.ecolind.2019.106020
Li ZH, Wang Q, Ruan X et al (2010) Phenolics and Plant Allelopathy. Mol 2010, Vol 15, Pages 8933–8952 15:8933–8952. https://doi.org/10.3390/MOLECULES15128933
Li H, Yao W, Liu Q et al (2017a) Application of UHPLC-ESI-Q-TOF-MS to identify multiple constituents in processed products of the herbal medicine ligustri lucidi fructus. Molecules 22. https://doi.org/10.3390/molecules22050689
Li N, Yang W, Fang S et al (2017b) Dispersal of invasive Phytolacca americana seeds by birds in an urban garden in China. Integr Zool 12:26–31. https://doi.org/10.1111/1749-4877.12214
Lorenzo P, Pazos-Malvido E, Rubido-Bará M et al (2012) Invasion by the leguminous tree Acacia dealbata (Mimosaceae) reduces the native understorey plant species in different communities. Aust J Bot 60:669. https://doi.org/10.1071/BT12036
Martínez-Fernández J, Hernández L, de la Vázquez A, Cañellas I (2012) (PDF) Distribución y dinámica del género Acacia en Galicia: un análisis a partir del Tercer y Cuarto Inventario Forestal Nacional. In: EEI 2012 Notas Científicas 4o Congreso Nacional sobre Especies Exóticas Invasoras. pp 128–132
Morsi MM, Abdelmigid HM (2016) Allelopathic activity of Eucalyptus globulus leaf aqueous extract on Hordeum vulgare growth and cytogenetic behaviour. AJCS 10:1835–2707. https://doi.org/10.21475/ajcs.2016.10.11.PNE122
Nelson KM, Bisbing S, Grossenbacher DL et al (2021) Testing an invasion mechanism for Eucalyptus globulus: is there evidence of allelopathy? Am J Bot 108:607–615. https://doi.org/10.1002/AJB2.1635
Novoa A, González L, Moravcová L, Pyšek P (2012) Effects of Soil characteristics, Allelopathy and Frugivory on Establishment of the Invasive Plant Carpobrotus edulis and a Co-Occuring native, Malcolmia littorea. PLoS ONE 7:e53166. https://doi.org/10.1371/JOURNAL.PONE.0053166
Nurjanah U, Setyowati N, Simarmata M (2020) Allelopathic potential of aqueous extract of Archidendron jiringa (Jering) pods for weed control in Swamp paddy field. Int J Agric Technol 16:1153–1164
Ohsaki H, Mukai H, Yamowo A (2020) Biochemical recognition in seeds: germination of Rumex obtusifolius is promoted by leaves of facilitative adult conspecifics. Plant Species Biol 35:233–242. https://doi.org/10.1111/1442-1984.12275
Orrock JL, Christopher CC (2010) Density of intraspecific competitors determines the occurrence and benefits of accelerated germination. Am J Bot 97:694–699. https://doi.org/10.3732/AJB.0900051
Pimentel D, Zuniga R, Morrison D (2005) Update on the environmental and economic costs associated with alien-invasive species in the United States. Ecol Econ 52:273–288. https://doi.org/10.1016/j.ecolecon.2004.10.002
Puig CG, Reigosa MJ, Valentão P et al (2018) Unravelling the bioherbicide potential of Eucalyptus globulus Labill: Biochemistry and effects of its aqueous extract. PLoS ONE 13:e0192872. https://doi.org/10.1371/JOURNAL.PONE.0192872
Pyšek P, Jarošík V, Hulme PE et al (2012) A global assessment of invasive plant impacts on resident species, communities and ecosystems: the interaction of impact measures, invading species’ traits and environment. Glob Chang Biol 18
Qu T, Du X, Peng Y et al (2021) Invasive species allelopathy decreases plant growth and soil microbial activity. PLoS ONE 16:e0246685. https://doi.org/10.1371/JOURNAL.PONE.0246685
Quirantes-Piné R, Lozano-Sánchez J, Herrero M et al (2013) HPLC-ESI-QTOF-MS as a powerful Analytical Tool for Characterising Phenolic Compounds in Olive-leaf extracts. Phytochem Anal 24:213–223. https://doi.org/10.1002/pca.2401
Reigosa MJ, Pazos-Malvido E (2007) Phytotoxic Effects of 21 plant secondary metabolites on Arabidopsis thaliana Germination and Root Growth. J Chem Ecol 33:1456–1466. https://doi.org/10.1007/S10886-007-9318-X
Reigosa MJ, Souto XC, González L (1999) Effect of phenolic compounds on the germination of six weeds species. Plant Growth Regul 28:83–88. https://doi.org/10.1023/A:1006269716762
Reigosa MJ, González L, Souto XC, Pastoriza JE (2000) Allelopathy in forest ecosystems. In: Allelopathy in Ecological Agriculture and Forestry. Springer Netherlands, pp 183–193
Renne IJ, Rios BG, Fehmi JS, Tracy BF (2004) Low allelopathic potential of an invasive forage grass on native grassland plants: a cause for encouragement? Basic Appl Ecol 5:261–269. https://doi.org/10.1016/J.BAAE.2003.11.001
Renne IJ, Sinn BT, Shook GW et al (2014) Eavesdropping in plants: delayed germination via biochemical recognition. J Ecol 102:86–94. https://doi.org/10.1111/1365-2745.12189
Reyes O, Casal M (1998) Germination of Pinus pinaster, P. radiata and Eucalyptus globulus in relation to the amount of ash produced in forest fires. Ann des Sci for 55:837–845. https://doi.org/10.1051/forest:19980707
Reyes O, Casal M, Trabaud L (1997) The influence of population, fire and time of dissemination on the germination of Betula pendula seeds. Plant Ecol 133:201–208. https://doi.org/10.1023/A:1009751513547
Riveiro SF, Cruz Ó, Casal M, Reyes O (2020) Fire and seed maturity drive the viability, dormancy, and germination of two invasive species: Acacia longifolia (Andrews) Willd. And Acacia mearnsii De Wild. Ann for Sci 77:60. https://doi.org/10.1007/s13595-020-00965-x
Romane F, Bacillieri R, Bran D, Bouchet MA (1992) Natural Degenerate Mediterranean Forests: Which Future ? The Examples of the Holm Oak (Quercus Ilex L.) and Chestnut (Castanea Sativa Mill.) Coppice Stands. In: Responses of Forest Ecosystems to Environmental Changes. Springer Netherlands, pp 374–380
Romero Buján MI (2007) Flora exótica de Galicia (noroeste ibérico). Bot Complut 31:113–125
Salgado J, Parajó JJ, Teijeira T et al (2017) New insight into the environmental impact of two imidazolium ionic liquids. Effects on seed germination and soil microbial activity. Chemosphere 185:665–672. https://doi.org/10.1016/j.chemosphere.2017.07.065
San-Miguel-Ayanz J, Moreno JM, Camia A (2013) Analysis of large fires in european Mediterranean landscapes: Lessons learned and perspectives. For Ecol Manage 294:11–22. https://doi.org/10.1016/J.FORECO.2012.10.050
Sanz-Elorza M, Dana ED, Sobrino-Vesperinas E (2004) Atlas de las plantas alóctonas invasoras de España. Dirección General para la Biodiversidad, Madrid
Simberloff D, von Holle B (1999) Positive Interactions of Nonindigenous Species: Invasional Meltdown? Biol Invasions 1999 11 1:21–32. https://doi.org/10.1023/A:1010086329619
Singh HP, Batish DR, Kohli RK (1999) Autotoxicity: Concept, organisms, and ecological significance. Taylor & Francis Group
Souto XC, González L, Reigosa MJ (1994) Comparative analysis of allelopathic effects produced by four forestry species during decomposition process in their soils in Galicia (NW Spain). J Chem Ecol 20:3005–3015. https://doi.org/10.1007/BF02098405
Souto XC, Bolaño JC, González L, Reigosa MJ (2001) Allelopathic effects of tree species on some soil microbial populations and herbaceous plants. Biol Plant 44:269–275. https://doi.org/10.1023/A:1010259627812
Stohlgren TJ, Barnett DT, Kartesz JT (2003) The rich get richer: patterns of plant invasions in the United States. Front Ecol Environ. https://doi.org/10.1890/1540-9295(2003)001[0011:TRGRPO]2.0.CO;2 1:
Stohlgren TJ, Jarnevich C, Chong GW, Evangelista PH (2006) Scale and plant invasions: a theory of biotic acceptance Měřítko studia a rostlinné invaze: teorie biotické akceptance. Preslia 78:405–426
Suresh KK, Vinaya Rai RS (1987) Studies on the allelopathic effects of some agroforestry tree crops. Int Tree Crop J 4:109–115. https://doi.org/10.1080/01435698.1987.9752816
Teerarak M, Laosinwattana C, Charoenying P (2010) Evaluation of allelopathic, decomposition and cytogenetic activities of Jasminum officinale L. f. var. Grandiflorum (L.) Kob. On bioassay plants. Bioresour Technol 101:5677–5684. https://doi.org/10.1016/J.BIORTECH.2010.02.038
Tielbörger K, Prasse R (2009) Do seeds sense each other? Testing for density-dependent germination in desert perennial plants. Oikos 118:792–800. https://doi.org/10.1111/J.1600-0706.2008.17175.X
Underwood EC, Klinger RC, Brooks ML (2019) Effects of invasive plants on fire regimes and postfire vegetation diversity in an arid ecosystem. Ecol Evol 9:12421–12435. https://doi.org/10.1002/ECE3.5650
Valdés B, Melero D, Girón V (2011) Plantas americanas naturalizadas en el territorio de Doñana (SO de la Península Ibérica). Lagascalia 31:7–20
Vilà M, Espinar JL, Hejda M et al (2011) Ecological impacts of invasive alien plants: a meta-analysis of their effects on species, communities and ecosystems. Ecol Lett 14:702–708
Wohlgemuth T, Gossner MM, Campagnaro T et al (2022) Impact of non-native tree species in Europe on soil properties and biodiversity: a review. NeoBiota 78:45–69. https://doi.org/10.3897/NEOBIOTA.78.87022
Yamowo A, Mukai H (2017) Seeds integrate biological information about conspecific and allospecific neighbours. Proc R Soc B Biol Sci 284:20170800. https://doi.org/10.1098/rspb.2017.0800
Yannelli FA, Novoa A, Lorenzo P et al (2020) No evidence for novel weapons: biochemical recognition modulates early ontogenetic processes in native species and invasive acacias. Biol Invasions 22:549–562. https://doi.org/10.1007/S10530-019-02110-W/FIGURES/3
Young AM, Larson BMH (2011) Clarifying debates in invasion biology: a survey of invasion biologists. Environ Res 111:893–898. https://doi.org/10.1016/j.envres.2011.06.006
Zhang Z, Liu Y, Yuan L et al (2021) Effect of allelopathy on plant performance: a meta-analysis. Ecol Lett 24:348–362. https://doi.org/10.1111/ELE.13627
Acknowledgements
The authors thank the financial support of the Spanish Ministry of Science, Innovation and Universities, the Castilla y León Regional Government, the Galicia Regional Government and the European Regional Development Fund (ERDF) trough FIRESEVES (AGL2017-86075-C2-2-R) and WUIFIRECYL (LE005P20) projects, the Competitive Reference Group BIOAPLIC (ED431C2019/07) and the Strategic Researcher Cluster BioReDeS (ED431E 2018/09). S. F. Riveiro is financially supported by a PhD Fellowship-Contract (ED481A-2020/088) from Xunta de Galicia (Axudas de apoio á etapa predoutoral 2020 - Modalidade B). Ó. Cruz is financially supported by a Collaboration agreement between Xunta de Galicia and University of Santiago de Compostela which regulates the “Campus Terra” Specialization Campus. Authors would like to thank the use of RIAIDT-USC analytical facilities. The authors would like to thank the time dedicated by Mercedes Uscola (Associate Editor) and Ian J. Renne (Reviewer) to carefully review the first version of the manuscript, their comments were very valuable and contributed to considerably improve the final version of the manuscript.
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare they have no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Riveiro, S.F., Cruz, Ó. & Reyes, O. Are the invasive Acacia melanoxylon and Eucalyptus globulus drivers of other species invasion? Testing their allelochemical effects on germination. New Forests 55, 751–767 (2024). https://doi.org/10.1007/s11056-023-10001-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11056-023-10001-1