Abstract
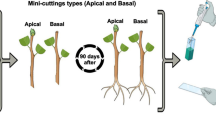
Eucalyptus globulus Labill and hybrids thereof have low lignin content, favoring cellulose extraction, but are often recalcitrant to clonal propagation. This work analyzed biochemical and morphological changes during adventitious rooting of mini-cuttings of E. globulus × maidenni obtained from mini-stumps cultured in drip fertigated sand bed or intermittent flooding tray commercial propagation systems. Morphological (% rooting, root number and length, mean rooting time) and biochemical parameters (peroxidase activity, total phenolic content and flavonoid content) were monitored to characterize the rooting phases. All of the rooting parameters were equivalent in both systems, indicating comparable efficiency of both methods in clonal propagation. Kinetic profiles of biochemical parameters were also similar, although the activity of peroxidases was an order of magnitude higher and the phenolic content about three times lower in cuttings derived from intermittent flooding-grown mini-stumps than in those derived from sand bed-grown mini-stumps. Taken together, results suggest that rooting phases were similar in both systems: induction before day 5, formation from day 5 to 15, and elongation from day 15 to 45. These data may contribute to the development of rooting phase-specific mineral nutrient solutions to maximize clonal propagation and plant survival.




Similar content being viewed by others
References
Assis TF, Fett-Neto AG, Alfenas AC (2004) Current techniques and prospects for the clonal propagation of hardwood with emphasis on Eucalyptus. In: Walter C, Carson M (eds) Plantation forest biotechnology for the 21th century, 1st edn. Research Sign Post, New Delhi
Berthon JY, Battraw MJ, Gaspar T, Boyer N (1993) Early test using phenolic compounds and peroxidase activity to improve in vitro rooting of Sequoiadendron giganteum (Lindl.). Buchholz Saussurea 27:7
Biemelt S, Keetman U, Albrecht G (1998) Re-aeration following hypoxia or anoxia leads to the activation of the antioxidant defense system in roots of wheat seedlings. Plant Physiol 116:651–658. doi:10.1104/pp.116.2.651
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein using the principles of protein dye binding. Anal Biochem 72:245–248. doi:10.1016/0003-2697(76)90527-3
Caboni E, Tonelli MG, Lauri P, Iacovacci P, Kevers C, Damiano C et al (1997) Biochemical aspects of almond microcuttings related to in vitro rooting ability. Biol Plant 39(1):91–97. doi:10.1023/A:1000365224324
Curir P, VanSumere CF, Termini A, Barthe P, Marchesini A, Dolci M (1990) Flavonoid accumulation is correlated with adventitious roots formation in Eucalyptus gunni Hook micropropagated through axillary bud stimulation. Plant Physiol 92:1148–1153
De Abreu IN, Mazzafera P (2005) Effect of water and temperature stress on the content of active constituents of Hypericum brasiliense Choisy. Plant Physiol Biochem 43:241–248. doi:10.1016/j.plaphy.2005.01.020
De Klerk GJ, Van Der Krieken W, De Jong JC (1999) The formation of adventitious roots: new concepts, new possibilities. In Vitro Cell Dev Biol Plant 35(3):189–199. doi:10.1007/s11627-999-0076-z
FAO (2000) Global forest resources assesment 2000–Main report. FAO Forestry paper. (www.fao.org/forestry/fo/fra/main/index.jsp)
Fett-Neto AG, Teixeira SL, Da Silva EAM, Sant’Anna R (1992) Biochemical and morphological changes during in vitro rhizogenesis in cuttings of Sequoia sempervirens (D. Don) EndI. J Plant Physiol 140:720–728
Fett-Neto AG, Fett JP, Goulart LWV, Pasquali G, Termignoni RR, Ferreira AG (2001) Distinct effects of auxin and light on adventitious root development in Eucalyptus saligna and Eucalyptus globulus. Tree Physiol 21:457–464
Garnczarska M, Bednarski W, Morkunas I (2004) Re-aeration-induced oxidative stress and antioxidative defenses in hypoxically pretreated lupine roots. J Plant Physiol 161:415–422. doi:10.1078/0176-1617-01073
Gaspar T, Kevers C, Hausman JF, Berthon JY, Ripetti V (1992) Practical uses of peroxidase activity as a predictive marker of rooting performance of micropropagated shoots. Agronomie 12:757–765. doi:10.1051/agro:19921003
Hand P (1993) Biochemical and molecular markers of cellular competence for adventitious rooting. In: Davis TD, Haissig BE (eds) Biology of adventitious root formation. Basic life sciences, v. 62. Plenum Press, New York
Hatzilazarou SP, Syros TD, Yupsanis TA, Bosabalidis AM, Economou AS (2006) Peroxidases, lignin and anatomy during in vitro and ex vitro rooting of gardenia (Gardenia jasminoides Ellis) microshoots. J Plant Physiol 163:827–836. doi:10.1016/j.jplph.2005.06.018
Husen A, Pal M (2007) Metabolic changes during adventitious root primordium development in Tectona grandis Linn. f. (teak) cuttings as affected by age of donor plants and auxin (IBA and NAA) treatment. New For 33:309–323. doi:10.1007/s11056-006-9030-7
Kevers C, Hausman JF, Faivre-Rampant O, Evers D, Gaspar T (1997) Hormonal control of adventitious rooting: progress and questions. J Appl Bot–Ang Bot 71(3–4):71–79
Le Roux JJ, Van Staden J (1991) Micropropagation and tissue culture of Eucalyptus–a review. Tree Physiol 9:435–477
Metaxas DJ, Syros TD, Yupsanis T, Economou AS (2004) Peroxidases during adventitious rooting in cuttings of Arbutus unedo and Taxus baccata as affected by plant genotype and growth regulator treatment. Plant Growth Regul 44:257–266. doi:10.1007/s10725-004-5931-7
Mora AL, Garcia CH (2000) A cultura do eucalipto no Brasil. Sociedade Brasileira de Silvicultura, São Paulo, Brazil
Nag S, Saha K, Choudhuri MA (2001) Role of auxin and polyamines in adventitious root formation in relation to changes in compounds involved in rooting. J Plant Growth Regul 20:182–194. doi:10.1007/s003440010016
Peer WA, Bandyopadhyay A, Blakeslee JJ, Makam SN, Chen RJ, Masson PH et al (2004) Variation in expression and protein localization of the PIN family of auxin efflux facilitator proteins in flavonoid mutants with altered auxin transport in Arabidopsis thaliana. Plant Cell 16:1898–1911. doi:10.1105/tpc.021501
Qaddoury A, Amssa M (2004) Effect of exogenous indole butyric acid on root formation and peroxidase and indole-3-acetic acid oxidase activities and phenolic contents in date palm offshoots. Bot Bull Acad Sin 45:127–131
Rival A, Bernard F, Mathieu Y (1997) Changes in peroxidase activity during in vitro rooting of oil palm (Elaeis guineensis Jacq). Sci Hortic (Amsterdam) 71:103–112. doi:10.1016/S0304-4238(97)00079-4
Rout GR, Samantaray S, Das P (2000) In vitro rooting of Psoralea corylifolia Linn: peroxidase activity as a marker. Plant Growth Regul 30:215–219. doi:10.1023/A:1006336819887
Saxena C, Samantaray S, Rout GR, Das P (2000) Effect of auxins on in vitro rooting of Plumbago zeylanica: peroxidase activity as a marker for root induction. Biol Plant 43(1):121–124. doi:10.1023/A:1026519417080
Schwambach J, Fadanelli C, Fett-Neto AG (2005) Mineral nutrition and adventitious rooting in microcuttings of Eucalyptus globulus. Tree Physiol 25:487–494
Serrano L, Rochange F, Semblant JP, Marque C, Teulières C, Boudet AM (1996) Genetic transformation of Eucalyptus globulus through biolistics: complementary development of procedures for organogenesis from zygotic embryos and stable transformation of corresponding proliferating tissue. J Exp Bot 45:285–290. doi:10.1093/jxb/47.2.285
Sokal RR, Rohlf FJ (1981) Biometry. W.H. Freeman, San Francisco, 859 p
Syros T, Yupsanis T, Zafiriadis H, Economou A (2004) Activity and isoforms of peroxidases, lignin and anatomy, during adventitious rooting in cuttings of Ebenus cretica L. J Plant Physiol 161:69–77. doi:10.1078/0176-1617-00938
Turnbull JW (1999) Eucalypt plantations. New For 17:37–52. doi:10.1023/A:1006524911242
Wasson AP, Pellerone FI, Mathesius U (2006) Silencing the flavonoid pathway in Medicago truncatula inhibits root nodule formation and prevents auxin transport regulation by rhizobia. Plant Cell 18:1617–1629. doi:10.1105/tpc.105.038232
Zhishen J, Mengcheng T, Jianming W (1999) The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem 64:555–559. doi:10.1016/S0308-8146(98)00102-2
Acknowledgments
The authors thank Aracruz Celulose S.A. for the supply of plant material and for the grant-in-aid of research to carry out this investigation. The Brazilian government agencies CAPES (Federal Agency for Graduate Education) and CNPq (National Council for Scientific and Technological Development) provided graduate, undergraduate and research scholarships.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schwambach, J., Ruedell, C.M., de Almeida, M.R. et al. Adventitious rooting of Eucalyptus globulus × maidennii mini-cuttings derived from mini-stumps grown in sand bed and intermittent flooding trays: a comparative study. New Forests 36, 261–271 (2008). https://doi.org/10.1007/s11056-008-9099-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11056-008-9099-2