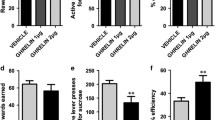
The effects of the ghrelin receptor antagonist [D-Lys3]-GHRP-6 on elements of gambling behavior and monoamine metabolism in the brain in rats were studied. A feeding reflex was developed in male rats over 21 days in a three-arm maze. The reward consisted of sunflower seeds. During the first five days, the animal received one seed at the end of arm 1, two seeds at the end of arm 2, and three seeds at the end of arm 3. The reinforcement delivery regime was then altered: in arm 1, each entry by the rat continued to yield one seed, while only every second entry into arm 2 yielded two seeds and only every third entry into arm 3 yielded three seeds. Thus, reward size on visiting arm 1 was minimal and while the probability of receiving it was maximal. Visiting arm 3 produced the maximal reward but with the lowest probability. Rats acquiring the stable skill of entering the maze were given intranasal 0.9% NaCl solution or [D-Lys3]-GHRP-6 and their behavior was evaluated. Three days after behavioral testing on the background of drug, animals were given 0.9% NaCl or [D-Lys3]-GHRP-6. Rats were decapitated 80 min later and the hypothalamus, hippocampus, striatum, and olfactory bulb (a component of the ventral striatum) were harvested from brains and HPLC assays were used to estimate contents of dopamine, serotonin, and their metabolites. The ghrelin receptor antagonist [D-Lys3]-GHRP-6 (20 μl, 1 mg/ml, intranasally) increased the number of excursions into maze arm 1 (with the highest probability but smallest reward). The substance had no effect on measures of dopamine and serotonin metabolism in the hypothalamus. [D-Lys3]-GHRP-6 significantly increased serotonin content in the left hippocampus. Measures of monoamine metabolism in the olfactory bulb and striatum changed only in the right hemisphere, with increases in 5-hydroxyindoleacetic acid: serotonin ratio in both structures. In the right striatum, these changes were also accompanied by increases in serotonin and dopamine metabolite contents. Thus, the ability of [D-Lys3]-GHRP-6 to change selection strategy in favor of the highest probability but smallest reinforcement is based on age-related activity of the dopaminergic and serotoninergic systems in the dorsal and ventral striatum of the right hemisphere of the brain and serotonin content in the left hippocampus.
Similar content being viewed by others
References
A. Blaszczynski, P. Collins, D. Fong, et al., “Responsible gambling: general principles and minimal requirements,” J. Gambl. Stud., 27, No. 4, 565–573 (2011), https://doi.org/10.1007/s10899-010-9214-0.
N. M. Petry, “Patterns and correlates of Gamblers Anonymous attendance in pathological gamblers seeking professional treatment,” Addict. Behav., 28, 1049–1062 (2003), https://doi.org/10.1016/s0306-4603(02)00233-2.
J. E. Grant, B. L. Odlaug, and L. R. N. Schreiber, “Pharmacological treatments in pathological gambling,” Br. J. Clin. Pharmacol., 77, No. 2, 375–381 (2014), https://doi.org/10.1111/j.1365-2125.2012.04457.x.
F. D. Zeeb, T. W. Robbins, and C. A. Winstanley, “Serotonergic and dopaminergic modulation of gambling behavior as assessed using a novel rat gambling task,” Neuropsychopharmacology, 34, No. 10, 2329–2343 (2009), https://doi.org/10.1038/npp.2009.62.
A. A. Lebedev, N. D. Yakushina, K. E. Gramota, et al., “Modeling of gambling in rats in the situation of choosing the strength and the probability of food reinforcement in a maze,” Narkologiya, 17, No. 5, 31–35 (2018), https://doi.org/10.25557/1682-8313.2018.05.31-35.
P. D. Shabanov, N. D. Yakushina, and A. A. Lebedev, “Pharmacology of peptide mechanisms of play behavior in rats,” Vopr. Narkol., 187, No. 4, 24–44 (2020), https://doi.org/10.47877/0234-0623_2020_4_24.
A. A. Lebedev, Yu. N. Bessolova, N. S. Efimov, et al., “Role of orexin peptide system in emotional overeating induced by brain reward stimulation in fed rats,” Res. Results Pharmacol., 6, No. 1, 81–91 (2020), https://doi.org/10.3897/rrpharmacology6.52180.
A. A. Lebedev, P. P. Khokhlov, N. D. Yakushina, et al., “Pharmacological and biochemical analysis of the involvement of the ghrelin peptide system in behavioral manifestations of gambling in rats,” Eksper. Klin. Farmakol., 82, No. 6, 16–20 (2019).
K. Wallin-Miller, G. Li, D. Kelishani, and R. I. Wood, “Anabolicandrogenic steroids alter decision making in a balanced rodent model of the Iowa gambling task,” Neuroscience, 132, No. 3, 152–160 (2018), https://doi.org/10.1037/bne0000243.
M. Kojima, H. Hosoda, Y. Date, et al., “Ghrelin is a growth-hormonereleasing acylated peptide from stomach,” Nature, 402, No. 6762, 656–660 (1999), https://doi.org/10.1038/45230.
Y. Yin, “The growth hormone secretagogue receptor: Its intracellular signaling and regulation,” Int. J. Mol. Sci., 15, No. 3, 4837–4855 (2014), https://doi.org/10.3390/ijms15034837.
P. D. Shabanov, A. A. Lebedev, E. R. Bychkov, et al., “Neurochemical mechanisms and pharmacology of ghrelins,” Obzory Klin. Farmakol. Lekarstv. Ter., 18, No. 1, 5–22 (2020), https://doi.org/10.17816/RCF1815-22.
J. L. Gomez, C. L. Cunningham, and D. A. Finn, “Differential effects of ghrelin antagonists on alcohol drinking and reinforcement in mouse and rat models of alcohol dependence,” Neuropharmacology, 97, No. 10, 182–193 (2015), https://doi.org/10.1016/j.neuropharm.2015.05.026.
Z. B. Andrews, “The extra-hypothalamic actions of ghrelin on neuronal function,” Trends Neurosci., 34, No. 1, 31–40 (2011), https://doi.org/10.1016/j.tins.2010.10.00121035199.
A. Kern, R. Albarran-Zeckler, H. E. Walsh, and R. G. Smith, “Apoghrelin receptor forms heteromers with DRD2 in hypothalamic neurons and is essential for anorexigenic effects of DRD2 agonism,” Neuron, 73, No. 2, 317–332 (2012), https://doi.org/10.1016/j.neuron.2011.10.038.
G. D. Rosen, S. Finklestein, A. L. Stoll, et al., “Neurochemical asymmetries in the albino rat’s cortex, striatum, and nucleus accumbens,” Life Sci., 34, No. 12, 1143–1148 (1984), https://doi.org/10.1016/0024-3205(84)90085-7.
G. Paxinos and C. Watson, The Rat Brain in Stereotaxic Coordinates, Academic Press, San Diego (2007); 6th edition.
I. N. Krasnova, E. R. Bychkov, V. I. Lioudyno, et al., “Intracerebroventricular administration of substance P increases dopamine content in the brain of 6-hydroxydopamine-lesioned rats,” Neuroscience, 95, No. 1, 113–117 (2000), https://doi.org/10.1016/s0306-4522(99)00400-5.
E. Pittaras, J. Callebert, M. Chennaoui, et al., “Individual behavioral and neurochemical markers of unadapted decision-making processes in healthy inbred mice,” Brain Struct. Funct., 221, No. 9, 4615–4629 (2016), https://doi.org/10.1007/s00429-016-1192-2.
B. de Laat, A. Weerasekera, G. Leurquin-Sterk, et al., “Glutamatergic biomarkers for cocaine addiction: a longitudinal study using MR spectroscopy and mGluR5 PET in self-administering rats,” J. Nucl. Med., 59, No. 6, 952–959 (2018), https://doi.org/10.2967/jnumed.117.202614.
P. V. Simonov (ed.), The Emotional Brain, Nauka, Moscow (1981).
D. Marazziti, F. Golia, M. Picchetti, et al., “Decreased density of the plate let serotonin transporter in pathological gamblers,” Neuropsychobiology, 57, No. 1–2, 38–43 (2008), https://doi.org/10.1159/000129665.
Y. Paloyelis, P. Asherson, M. A. Mehta, et al., “DAT1 and COMT effects on delay discounting and trait impulsivity in male adolescents with attention defi cit/hyperactivity disorder and healthy controls,” Neuropsychopharmacology, 35, No. 12, 2414–2426 (2010), https://doi.org/10.1038/npp.2010.124.
K. L. Hanson, M. Luciana, and K. Sullwold, “Reward-related decision- making deficits and elevated impulsivity among MDMA and other drug users,” Drug Alcohol Depend., 96, No. 1–2, 99–110 (2008), https://doi.org/10.1016/j.drugalcdep.2008.02.003.
S. F. Stoltenberg and J. M. Vandever, “Gender moderates the association between 5-HTTLP Rand decision-making under ambiguity but not under risk,” Neuropharmacology, 58, No. 2, 423–428 (2010), https://doi.org/10.1016/j.neuropharm.2009.09.010.
J. R. Homberg and K. P. Lesch, “Looking on the bright side of serotonin transporter gene variation,” Biol. Psychiatry, 69, No. 6, 513–519 (2010), https://doi.org/10.1016/j.biopsych.2010.09.024.
M. Mimura, R. Oeda, and M. Kawamura, “Impaired decision-making in Parkinson’s disease,” Parkinsonism Relat. Disord., 12, No. 3, 169–175 (2006), https://doi.org/10.1016/j.parkreldis.2005.12.003.
J. Linnet, A. Moller, E. Peterson, et al., “Dopamine release in ventral striatum during Iowa gambling task performance is associated with increased excitement levels in pathological gambling,” Addiction, 106, No. 2, 383–390 (2011), https://doi.org/10.1111/j.1360-0443.2010.03126.x.
R. van den Bos, J. Homberg, E. Gijsbers, et al., “The effect of COMT Val158Met genotype on decision-making and preliminary findings on its interaction with the 5-HTTLPR in healthy females,” Neuropharmacology, 56, No. 2, and K. 493–498 (2009), https://doi.org/10.1016/j.neuropharm.2008.10.002.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Rossiiskii Fiziologicheskii Zhurnal imeni I. M. Sechenova, Vol. 107, No. 9, pp. 1100–1111, September, 2021.
Rights and permissions
About this article
Cite this article
Lebedev, A.A., Karpova, I.V., Bychkov, E.R. et al. The Ghrelin Antagonist [D-Lys3]-GHRP-6 Decreases Signs of Risk Behavior in a Model of Gambling Addiction in Rats by Altering Dopamine and Serotonin Metabolism. Neurosci Behav Physi 52, 415–421 (2022). https://doi.org/10.1007/s11055-022-01255-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11055-022-01255-x