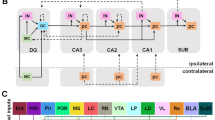
The θ rhythm (4–12 Hz) in the main rhythm in the hippocampus and in contrast to other rhythms it has its own pacemaker (the medial septal area) and physiological regulatory mechanisms. Generation of the θ rhythm is critically required for processing novel information arriving in the hippocampus. This review seeks to describe current views of how synchronization of the neural network of the hippocampus at the θ rhythm is involved in cognitive processes.
Similar content being viewed by others
References
Alger, B. E. and Teyler, T. J., “Long-term and short-term plasticity in the CA1, CA3, and dentate regions of the rat hippocampal slice,” Brain Res., 110, 463–480 (1976).
Astasheva, E., Astashev, M., and Kitchigina, V., “Changes in the behavior and oscillatory activity in cortical and subcortical brain structures induced by repeated l-glutamate injections to the medial septal area in guinea pigs,” Epilepsy Res., 109, 134–145 (2015).
Belluscio, M. A., Mizuseki, K., Schmidt, R., et al., “Cross-frequency phase-phase coupling between θ and γ oscillations in the hippocampus,” J. Neurosci., 32, 423–435 (2012).
Bi, G. and Poo, M., “Distributed synaptic modification in neural networks induced by patterned stimulation,” Nature, 401, 792–796 (1999).
Bi, G. Q. and Poo, M. M., “Synaptic modifications in cultured hippocampal neurons: dependence on spike timing, synaptic strength, and postsynaptic cell type,” J. Neurosci., 18, 10,464–10,472 (1998).
Bittner, K. C., Milstein, A. D., Grienberger, C., et al., “Behavioral time scale synaptic plasticity underlies CA1 place fields,” Science, 357, 1033–1036 (2017).
Brazhnik, E. S., “Theta rhythmicity in the medial septum: entraining by the GABA-ergic neurons,” Zh. Vyssh. Nerv. Deyat., 54, No. 2, 192–201 (2004).
Brzosko, Z., Zannone, S., Schultz, W., et al., “Sequential neuromodulation of Hebbian plasticity offers mechanism for effective reward-based navigation,” eLife, 6, 0–0 (2017).
Burak, Y. and Fiete, I. R., “Accurate path integration in continuous attractor network models of grid cells,” PLoS Comput. Biol., 5, e1000291 (2009).
Burak, Y., “Spatial coding and attractor dynamics of grid cells in the entorhinal cortex,” Curr. Opin. Neurobiol., 25, 169–175 (2014).
Burgess, A. P. and Gruzelier, J. H., “Short duration synchronization of human theta rhythm during recognition memory,” Neuroreport, 8, 1039–1042 (1997).
Burgess, C. P. and Burgess, N., “Controlling phase noise in oscillatory interference models of grid cell firing,” J. Neurosci., 34, 6224–6232 (2014).
Burgess, N., “Grid cells and theta as oscillatory interference: theory and predictions,” Hippocampus, 18, 1157–1174 (2008).
Burgess, N., Barry, C., and O’Keefe, J., “An oscillatory interference model of grid cell firing,” Hippocampus, 17, 801–812 (2007).
Bush, D. and Burgess, N., “A hybrid oscillatory interference/continuous attractor network model of grid cell firing,” J. Neurosci., 34, 5065–5079 (2014).
Buzsáki, G. and Moser, E. I., “Memory, navigation and theta rhythm in the hippocampal-entorhinal system,” Nat. Neurosci., 16, 130–138 (2013).
Buzsáki, G., “Hippocampal sharp wave-ripple: A cognitive biomarker for episodic memory and planning,” Hippocampus, 25, 1073–1188 (2015).
Buzsáki, G., “Theta oscillations in the hippocampus,” Neuron, 33, 325–340 (2002).
Buzsáki, G., “Two-stage model of memory trace formation: a role for, ‘noisy’ brain states,” Neuroscience, 31, 551– (1989).
Buzsáki, G., Anastassiou, C. A., and Koch, C., “The origin of extracellular fields and currents-EEG, ECoG, LFP and spikes,” Nat. Rev. Neurosci., 13, 407–420 (2012).
Buzsáki, G., Horváth, Z., Urioste, R., et al., “High-frequency network oscillation in the hippocampus,” Science, 256, 1025–1027 (1992).
Carr, M. F., Jadhav, S. P., and Frank, L. M., “Hippocampal replay in the awake state: a potential substrate for memory consolidation and retrieval,” Nat. Neurosci., 14, 147–153 (2011).
Chaieb, L., Leszczynski, M., Axmacher, N., et al., “Theta-gamma phase-phase coupling during working memory maintenance in the human hippocampus,” Cogn. Neurosci., 6, 149–157 (2015).
Chen, Z., Resnik, E., McFarland, J. M., et al., “Speed controls the amplitude and timing of the hippocampal gamma rhythm,” PLoS One, 6, e21408 (2011).
Chizhov, A. V., Sanchez-Aguilera, A., Rodrigues, S., and de la Prida, L. M., “Simplest relationship between local field potential and intracellular signals in layered neural tissue,” Phys. Rev. E Stat. Nonlin. Soft Matter Phys., 92, 062704 (2015).
Couey, J. J., Witoelar, A., Zhang, S. J., et al., “Recurrent inhibitory circuitry as a mechanism for grid formation,” Nat. Neurosci., 16, 318–324 (2013).
Csicsvari, J., Hirase, H., Czurkó, A., et al., “Oscillatory coupling of hippocampal pyramidal cells and interneurons in the behaving rat,” J. Neurosci., 19, 274–287 (1999).
Cutsuridis, V. and Poirazi, P., “A computational study on how theta modulated inhibition can account for the long temporal windows in the entorhinal-hippocampal loop,” Neurobiol. Learn. Mem., 120, 69–83 (2015).
Cutsuridis, V., Cobb, S., and Graham, B. P., “Encoding and retrieval in a model of the hippocampal CA1 microcircuit,” Hippocampus, 20, 423–446 (2010).
D’Albis, T., Jaramillo, J., Sprekeler, H., and Kempter, R., “Inheritance of hippocampal place fields through hebbian learning: effects of theta modulation and phase precession on structure formation,” Neural Comput., 27, 1624–1672 (2015).
Diekelmann, S. and Born, J., “The memory function of sleep,” Nat. Rev. Neurosci., 11, 114–126 (2010).
Doppelmayr, M., Klimesch, W., Schwaiger, J., et al., “Theta synchronization in the human EEG and episodic retrieval,” Neurosci. Lett., 257, 41–44 (1998).
Eichenbaum, H., “Time cells in the hippocampus: a new dimension for mapping memories,” Nat. Rev. Neurosci., 15, 732–744 (2014).
Feldman, D. E., “The spike-timing dependence of plasticity,” Neuron, 75, 556–571 (2012).
Foster, D. J. and Wilson, M. A., “Hippocampal theta sequences,” Hippocampus, 17, 1093–1099 (2007).
Freund, T. F. and Buzsáki, G., “Interneurons of the hippocampus,” Hippocampus, 6, 347–470 (1996).
Fries, P., “A mechanism for cognitive dynamics: neuronal communication through neuronal coherence,” Trends Cogn. Sci., 9, 474–480 (2005).
Fuhs, M. C. and Touretzky, D. S., “A spin glass model of path integration in rat medial entorhinal cortex,” J. Neurosci., 26, 4266–4276 (2006).
Gołebiewski, H., Eckersdorf, B., and Konopacki, J., “Electrical coupling underlies theta rhythm in freely moving cats,” Eur. J. Neurosci., 24, 1759–1770 (2006).
Grastyan, E., Lissak, K., Madarasz, I., and Donhoffer, H., “Hippocampal electrical activity during the development of conditioned reflexes,” Electroencephalogr. Clin. Neurophysiol., 11, 409–430 (1959).
Green, J. D. and Arduini, A. A., “Hippocampal electrical activity in arousal,” J. Neurophysiol, 17, 533–557 (1954).
Grunwald, M., Weiss, T., Krause, W., et al., “Theta power in the EEG of humans during ongoing processing in a haptic object recognition task,” Brain Res. Cogn. Brain Res., 11, 3337 (2001).
Hafting, T., Fyhn, M., Molden, S., et al., “Microstructure of a spatial map in the entorhinal cortex,” Nature, 436, 801–806 (2005).
Hartley, T., Lever, C., Burgess, N., and O’Keefe, J., “Space in the brain: how the hippocampal formation supports spatial cognition,” Philos. Trans. R. Soc. Lond. B Biol. Sci., 369, 20120510 (2014).
Hasselmo, M. E. and Brandon, M. P., “A model combining oscillations and attractor dynamics for generation of grid cell firing,” Front. Neural Circuits, 6, 30 (2012).
Hasselmo, M. E., “Neuromodulation and the hippocampus: memory function and dysfunction in a network simulation,” Prog. Brain Res., 121, 3–18 (1999).
Hasselmo, M. E., Bodelón, C., and Wyble, B. P., “A proposed function for hippocampal theta rhythm: separate phases of encoding and retrieval enhance reversal of prior learning,” Neural Comput., 14, 793–817 (2002).
Herculano-Houzel, S., “The human brain in numbers: a linearly scaled-up primate brain,” Front. Hum. Neurosci., 3, 31 (2009).
Jeewajee, A., Barry, C., Douchamps, V., et al., “Theta phase precession of grid and place cell firing in open environments,” Philos. Trans. R. Soc. Lond. B. Biol. Sci., 369, 20120532 (2014).
Jeewajee, A., Barry, C., O’Keefe, J., and Burgess, N., “Grid cells and theta as oscillatory interference: electrophysiological data from freely moving rats,” Hippocampus, 18, 1175–1185 (2008).
Jeffery, K. J., “Place cells, grid cells, attractors, and remapping,” Neural Plast., 2011, 182602 (2011).
Jouvet, M., “Biogenic amines and the states of sleep,” Science, 163, 32–41 (1969).
Jung, R. and Kornmüller, A. E., “Eine Methodik der Ableitung Iokalisierter Potentialschwankungen aus subcorticalen Hirngebieten,” Archiv. Psychiatrie, 109, 1–30 (1938).
Justus, D., Dalügge, D., Bothe, S., et al., “Glutamatergic synaptic integration of locomotion speed via septoentorhinal projections,” Nat. Neurosci., 20, 16–19 (2017).
Kelemen, E., Morón, I., and Fenton, A. A., “Is the hippocampal theta rhythm related to cognition in a non-locomotor place recognition task?” Hippocampus, 15, No. 4, 472–479 (2005).
Klausberger, T., Magill, P. J., Márton, L. F., et al., “Brain-state- and cell-type-specific firing of hippocampal interneurons in vivo,” Nature, 421, 844–848 (2003).
Klimesch, W., Doppelmayr, M., Yonelinas, A., et al., “Theta synchronization during episodic retrieval: neural correlates of conscious awareness,” Brain Res. Cogn. Brain Res., 12, 33–38 (2001).
Kocsis, B., Bragin, A., and Buzsáki, G., “Interdependence of multiple theta generators in the hippocampus: a partial coherence analysis,” J. Neurosci., 19, 6200–6212 (1999).
Kreiman, G., Koch, C., and Fried, I., “Category-specific visual responses of single neurons in the human medial temporal lobe,” Nat. Neurosci., 3, 946–953 (2000).
Ledberg, A. and Robbe, D., “Locomotion-related oscillatory body movements at 6–12 Hz modulate the hippocampal theta rhythm,” PLoS One, 6, e27575 (2011).
Lee, D. J., Izadi, A., Melnik, M., et al., “Stimulation of the medial septum improves performance in spatial learning following pilocarpine-induced status epilepticus,” Epilepsy Res., 130, 53–63 (2017).
Lisman, J. E. and Otmakhova, N. A., “Storage, recall, and novelty detection of sequences by the hippocampus: elaborating on the SOCRATIC model to account for normal and aberrant effects of dopamine,” Hippocampus, 11, 551–568 (2001).
Lisman, J., Grace, A. A., and Duzel, E., “A neoHebbian framework for episodic memory; role of dopamine-dependent late LTP,” Trends Neurosci., 34, 536–547 (2011).
Long, L. L., Hinman, J. R., Chen, C. M. A., et al., “Novel acoustic stimuli can alter locomotor speed to hippocampal theta relationship,” Hippocampus, 24, 1053–1058 (2014).
Lörincz, A. and Buzsáki, G., “Two-phase computational model training long-term memories in the entorhinal-hippocampal region,” Ann. N. Y. Acad. Sci., 911, 83–111 (2000).
Louie, K. and Wilson, M. A., “Temporally structured replay of awake hippocampal ensemble activity during rapid eye movement sleep,” Neuron, 29, 145–156 (2001).
Manns, J. R., Zilli, E. A., Ong, K. C., et al., “Hippocampal CA1 spiking during encoding and retrieval: relation to theta phase,” Neurobiol. Learn. Mem., 87, 9–20 (2007).
Markram, H., Lübke, J., Frotscher, M., and Sakmann, B., “Regulation of synaptic efficacy by coincidence of postsynaptic APs and EPSPs,” Science, 275, 213–215 (1997).
Marr, D., “A theory for cerebral neocortex,” Proc. Roy. Soc. Lond. B. Biol. Sci, 176, 161–234 (1970).
Mehta, M. R., “From synaptic plasticity to spatial maps and sequence learning,” Hippocampus, 25, 756–762 (2015).
Mitchell, S. J., Rawlins, J. N., Steward, O., and Olton, D. S., “Medial septal area lesions disrupt theta rhythm and cholinergic staining in medial entorhinal cortex and produce impaired radial arm maze behavior in rats,” J. Neurosci., 2, 292–302 (1982).
Mizumori, S. J., Perez, G. M., Alvarado, M. C., et al., “Reversible inactivation of the medial septum differentially affects two forms of learning in rats,” Brain Res., 528, 12–20 (1990).
Mizuseki, K., Royer, S., Diba, K., and Buzsáki, G., “Activity dynamics and behavioral correlates of CA3 and CA1 hippocampal pyramidal neurons,” Hippocampus, 22, 1659–1680 (2012).
Mizuseki, K., Sirota, A., Pastalkova, E., and Buzsáki, G., “Theta oscillations provide temporal windows for local circuit computation in the entorhinal-hippocampal loop,” Neuron, 64, 267–280 (2009).
Montgomery, S. M., Betancur, M. I., and Buzsáki, G., “Behavior-dependent coordination of multiple theta dipoles in the hippocampus,” J. Neurosci., 29, 1381–1394 (2009).
Moser, M. B., Rowland, D. C., and Moser, E. I., “Place cells, grid cells, and memory,” Cold Spring Harb. Perspect. Biol., 7, a021808 (2015).
Nádasdy, Z., Hirase, H., Czurkó, A., et al., “Replay and time compression of recurring spike sequences in the hippocampus,” J. Neurosci., 19, 9497–9507 (1999).
O’Keefe, J. and Recce, M. L., “Phase relationship between hippocampal place units and the EEG theta rhythm,” Hippocampus, 3, 317–330 (1993).
O’Keefe, J., “Place units in the hippocampus of the freely moving rat,” Exp. Neurol., 51, 78–109 (1976).
Patel, J., Fujisawa, S., Berényi, A., et al., “Traveling theta waves along the entire septotemporal axis of the hippocampus,” Neuron, 75, 410–417 (2012).
Petsche, H. and Stumpf, C., “The origin of theta-rhythm in the rabbit hippocampus,” Wien. Klin. Wochenschr., 74, 696–700 (1962).
Pickenhain, L. and Klingberg, F., “Changes of electrophysiological and behavioural indicators during the delay period of conditioned reflexes in rats,” Act. Nerv. Super. (Praha), 9, 330 (1967).
Ponulak, F. and Hopfield, J. J., “Rapid, parallel path planning by propagating wavefronts of spiking neural activity,” Front. Comput. Neurosci., 7, 98 (2013).
Preobrazhenskaya, L. A., “Dependence of the electrical activity of the hippocampus on the probability of reinforcement of an alimentary conditional stimulus,” Neurosci. Behav. Physiol., 21, 296–302 (1991).
Quiroga, R. Q., Reddy, L., Kreiman, G., et al., “Invariant visual representation by single neurons in the human brain,” Nature, 435, 1102–1107 (2005).
Robinson, J., Manseau, F., Ducharme, G., et al., “Optogenetic activation of septal glutamatergic neurons drive hippocampal theta rhythms,” J. Neurosci., 36, 3016–3023 (2016).
Robinson, T. E. and Whishaw, I. Q., “Effects of posterior hypothalamic lesions on voluntary behavior and hippocampal electroencephalograms in the rat,” J. Comp. Physiol. Psychol., 86, 768–786 (1974).
Sarnthein, J., Petsche, H., Rappelsberger, P., et al., “Synchronization between prefrontal and posterior association cortex during human working memory,” Proc. Natl. Acad. Sci. USA, 95, 7092–7096 (1998).
Si, B., Romani, S., and Tsodyks, M., “Continuous attractor network model for conjunctive position-by-velocity tuning of grid cells,” PLoS Comput. Biol., 10, e1003558 (2014).
Sinnamon, H. M., “Hippocampal theta activity and behavioral sequences in a reward-directed approach locomotor task,” Hippocampus, 15, 518–534 (2005a).
Sinnamon, H. M., “Hippocampal theta activity related to elicitation and inhibition of approach locomotion,” Behav. Brain Res., 160, 236–249 (2005b).
Skaggs, W. E. and McNaughton, B. L., “Replay of neuronal firing sequences in rat hippocampus during sleep following spatial experience,” Science, 271, 1870–1873 (1996).
Skaggs, W. E., McNaughton, B. L., Wilson, M. A., and Barnes, C. A., “Theta phase precession in hippocampal neuronal populations and the compression of temporal sequences,” Hippocampus, 6, 149–172 (1996).
Sokolov, E. N., “Higher nervous functions; the orienting reflex,” Annu. Rev. Physiol., 25, 545–580 (1963).
Solovyeva, K. P., Karandashev, I. M., Zhavoronkov, A., and Dunin-Barkowski, W. L., “Models of innate neural attractors and their applications for neural information processing,” Front. Syst. Neurosci., 9, 178 (2015).
Somogyi, P., Katona, L., Klausberger, T., et al., “Temporal redistribution of inhibition over neuronal subcellular domains underlies state-dependent rhythmic change of excitability in the hippocampus,” Philos. Trans. R. Soc. Lond. B. Biol. Sci., 369, 2012.0518 (2014).
Stella, F. and Treves, A., “Associative memory storage and retrieval: involvement of theta oscillations in hippocampal information processing,” Neural Plast., 2011, 683961 (2011).
Sullivan, D., Csicsvari, J., Mizuseki, K., et al., “Relationships between hippocampal sharp waves, ripples, and fast gamma oscillation: influence of dentate and entorhinal cortical activity,” J. Neurosci., 31, 8605–8616 (2011).
Sweet, J. A., Eakin, K. C., Munyon, C. N., and Miller, J. P., “Improved learning and memory with theta-burst stimulation of the fornix in rat model of traumatic brain injury,” Hippocampus, 24, 1592–1600 (2014).
Tesche, C. D. and Karhu, J., “Theta oscillations index human hippocampal activation during a working memory task,” Proc. Natl. Acad. Sci. USA, 97, 919–924 (2000).
Ulanovsky, N. and Moss, C. F., “Hippocampal cellular and network activity in freely moving echolocating bats,” Nat. Neurosci., 10, 224–233 (2007).
Vanderwolf, C. H., “Hippocampal electrical activity and voluntary movement in the rat,” Electroencephalogr. Clin. Neurophysiol., 26, 407–418 (1969).
Vanderwolf, C. H., “Neocortical and hippocampal activation relation to behavior: effects of atropine, eserine, phenothiazines, and amphetamine,” J. Comp. Physiol. Psychol., 88, 300–323 (1975).
Vertes, R. P., Hoover, W. B., and Viana Di Prisco, G., “Theta rhythm of the hippocampus: subcortical control and functional significance,” Behav. Cogn. Neurosci. Rev., 3, 173–200 (2004).
Vinogradova, O. S. and Dudaeva, K. I., “The comparator function of the hippocampus,” Dokl. Akad. Nauk. SSSR, 202, 241 (1972).
Vinogradova, O. S., “Expression, control, and probable functional significance of the neuronal theta-rhythm,” Prog. Neurobiol., 45, 523–583 (1995).
Vinogradova, O. S., “Hippocampus as comparator: role of the two input and two output systems of the Hippocampus in selection and registration of information,” Hippocampus, 11, 578–598 (2001).
Vinogradova, O. S., The Hippocampus and Memory, Nauka, Moscow (1975).
Wang, Y., Romani, S., Lustig, B., et al., “Theta sequences are essential for internally generated hippocampal firing fields,” Nat. Neurosci., 18, 282–288 (2015).
West, M. J. and Gundersen, H. J., “Unbiased stereological estimation of the number of neurons in the human hippocampus,” J. Comp. Neurol., 296, 1–22 (1990).
Wójtowicz, T. and Mozrzymas, J. W., “Diverse impact of neuronal activity at θ frequency on hippocampal long-term plasticity,” J. Neurosci. Res., 93, 1330–1344 (2015).
Wyble, B. P., Hyman, J. M., Rossi, C. A., and Hasselmo, M. E., “Analysis of theta power in hippocampal EEG during bar pressing and running behavior in rats during distinct behavioral contexts,” Hippocampus, 14, 662–674 (2004).
Yoshida, M. and Hayashi, H., “Emergence of sequence sensitivity in a hippocampal CA3–CA1 model,” Neural Netw., 20, 653–667 (2007).
Zhang, H. and Jacobs, J., “Traveling theta waves in the human hippocampus,” J. Neurosci., 35, 12477–12487 (2015).
Zutshi, I., Brandon, M. P., Fu, M. L., et al., “Hippocampal neural circuits respond to optogenetic pacing of theta frequencies by generating accelerated oscillation frequencies,” Curr. Biol., 28, 1179–1188.e3 (2018).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Zhurnal Vysshei Nervnoi Deyatel’nosti imeni I. P. Pavlova, Vol. 70, No. 3, pp. 314–325, May–June, 2020.
Rights and permissions
About this article
Cite this article
Mysin, I.E. The Functions of the Hippocampal θ Rhythm. Neurosci Behav Physi 50, 1176–1184 (2020). https://doi.org/10.1007/s11055-020-01019-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11055-020-01019-5