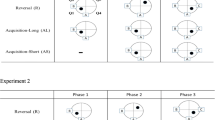
High-impulsivity (HI) rats learned to locate a visible platform bearing a special stimulus object (a bannerette) in the Morris water maze more quickly than low-impulsivity (LI) rats. HI rats also successfully acquired differentiation between a sail (the differential stimulus object) and the bannerette, as assessed in terms of a decrease in the number of incorrect swims to the location of the sail. During differentiation, HI rats reached the platform more quickly than LI rats and thus swam shorter distances. In the case of reverse differentiation (remodeling), between-group differences in platform reaching time and distance covered disappeared. However, the number of swims to the differential object (now the bannerette) by HI rats decreased, which did not occur in LI animals. These data provide evidence that “egocentric” tasks in the Morris water maze with the platform visible and bearing the bannerette are solved more easily by HI rats, while “allocentric” tasks associated with finding a platform hidden beneath the surface of the water are solved better by LI animals.
Similar content being viewed by others
References
Bañuelos, C., Gilbert, R. J., Montgomery, K. S., et al., “Altered spatial learning and delay discounting in a rat model of human third trimester binge ethanol exposure,” Behav. Pharmaco.l, 23, No. 1, 54–65 (2012).
Bénard, M., Bellisle, F., Kesse-Guyot, E., et al., “Impulsivity is associated with food intake, snacking, and eating disorders in a general population,” Am. J. Clin. Nutr., 109, No. 1, 117–126 (2019).
Blondel, A., Simon, H., Sanger, D. J., and Moser, P., “The effect of repeated nicotine administration on the performance of drug-naive rats in a five-choice serial reaction time task,” Behav. Pharmacol., 10, No. 6–7, 665–673 (1999).
Brown, H. E., Hart, K. L., Snapper, L. A., et al., “Impairment in delay discounting in schizophrenia and schizoaffective disorder but not primary mood disorders,” NPJ Schizophr., 4, No. 1, 9 (2018).
Canário, N., Sousa, M., Moreira, F., et al., “Impulsivity across reactive, proactive and cognitive domains in Parkinson’s disease on dopaminergic medication: Evidence for multiple domain impairment,” PLoS One, 14, No. 2, e0210880 (2019).
Cardinal, R. N., Winstanley, C. A., Robbins, T. W., and Everitt, B. J., “Limbic corticostriatal systems and delayed reinforcement,” Ann. N.Y. Acad. Sci., 1021, 33–50 (2004).
Carli, M., Robbins, T. W., Evenden, J. L., and Everitt, B. J., “Effects of lesions to ascending noradrenergic neurones on performance of a 5-choice serial reaction task in rats; implications for theories of dorsal noradrenergic bundle function based on selective attention and arousal,” Behav. Brain Res., 9, No. 3, 361–80 (1983).
Cheung, T. H. and Cardinal, R. N., “Hippocampal lesions facilitate instrumental learning with delayed reinforcement but induce impulsive choice in rats,” BMC Neurosci., 13, No. 6, 36 (2005).
Dellu-Hagedorn, F., “Relationship between impulsivity, hyperactivity and working memory: A differential analysis in the rat,” Behav. Brain Funct., 2, 10 (2006), https://doi.org/https://doi.org/10.1186/1744-9081-2-10.
Eagle, D. M. and Robbins, T. W., “Inhibitory control in rats performing a stop-signal reaction-time task: effects of lesions of the medial striatum and d-amphetamine,” Behav, Neurosci. Psychopharmacol., 117, No. 6, 1302–1317 (2003).
Ferguson, S. A. and Cada, A. M., “Spatial learning/memory and social and nonsocial behaviors in the spontaneously hypertensive, Wistar–Kyoto and Sprague–Dawley rat strains,” Pharmacol. Biochem. Behav., 77, No. 3, 583–594 (2004).
Higgins, G. A. and Silenieks, L. B., “Rodent test of attention task and impulsivity: the 5-choice serial reaction time,” Curr. Protoc. Pharmacol., 78, 5.49.1–5.49.34 (2017).
Izquierdo, A. and Jentsch, J. D., “Reversal learning as a measure of impulsive and compulsive behavior in addictions,” Psychopharmacology, 219, No. 2, 607–620 (2012).
Johnson, S. L., Carver, C. S., and Tharp, J. A., “Suicidality in bipolar disorder: The role of emotion-triggered impulsivity,” Suicide Life Threat. Behav., 47, No. 2, 177–192 (2017).
Levandovskaya, A. A., Zaichenko, M. I., and Merzhanova, G. Kh., “Assessment of exploratory activity and anxiety in rats with different levels of impulsive behavior,” Zh. Vyssh. Nerv. Deyat., 63, No. 6, 719–729 (2013).
Linhartová, P., Širůček, J., Ejova, A., et al., “Dimensions of impulsivity in healthy people, patients with borderline personality disorder, and patients with attention-deficit/hyperactivity disorder,” J. Atten. Disord., (2019), 10:1087054718822121 (2019).
Logan, G. D., Schachar, R. J., and Tannock, R., “Impulsivity and inhibitory control,” Psychol. Sci., 1, 60–64 (1997).
Mariano, T. Y., Bannerman, D. M., McHugh, S. B., et al., “Impulsive choice in hippocampal but not orbitofrontal cortex-le-sioned rats on a nonspatial decision-making maze task,” Eur. J. Neurosci., 3, 472–484 (2009).
Mazur, J., “An adjusting procedure for studying delayed reinforcementm,” in: Quantitative Analysis of Behaviour. The Effect of Delay and Intervening Events on Reinforcement Value, M. L. Commons, J. A. Nevin, and H. C. Rachlin (eds.), Erlbaum, Hillsdale, N.J. (1987), Vol. 56, pp. 55–73.
Mitchell, S. H., Napier, T. C., Reynolds, B., et al., “Choice impulsivity: Definitions, measurement issues, and clinical implications,” Personal. Disord., 6, No. 2, 182–198 (2015).
Patros, C. H., Alderson, R. M., Kasper, L. J., et al., “Choice-impulsivity in children and adolescents with attention deficit/hyperactivity disorder (ADHD): A meta-analytic review,” Clin. Psychol. Rev., 43, 162–174 (2016).
Renda, C. R., Stein, J. S., and Madden, G. J., “Impulsive choice predicts poor working memory in male rats, PloS One, 9, No. 4, e93263 (2014).
Robbins, T. W., “The 5-choice serial reaction time task: behavioral pharmacology and functional neurochemistry,” Psychopharmacology, 163, No. 3–4, 362–380 (2002).
Robinson, L., Bridge, H., and Riedel, G., “Visual discrimination learning in the water maze: a novel test for visual acuity,” Behav. Brain Res., 119, No. 1, 77–84 (2001).
Salamone, J. D., Cousins, M. S., and Bucher, S., “Anhedonia or anergia? Effects of haloperidol and nucleus accumbens dopamine depletion on instrumental response selection in a T-maze cost/benefit procedure,” Behav. Brain Res., 5, No. 2, 221–229 (1994).
Schoenbaum, G., Nugent S. L, Saddoris, M. P., and Setlow, B., “Orbitofrontal lesions in rats impair reversal but not acquisition of go, no-go odor discriminations,” Neuroreport, 13, No. 6, 885–890 (2002).
Sontag, T. A., Fuermaier, A. B., Hauser, J., et al., “Spatial memory in spontaneously hypertensive rats (SHR),” PLoS One, 8, No. 8, e74660 (2013).
Vaughan, C. L., Stangl, B. L., Schwandt, M. L., et al., “The relationship between impaired control, impulsivity, and alcohol self-administration in nondependent drinkers,” Exp. Clin. Psychopharmacol., (2019), https://doi.org/https://doi.org/10.1037/pha0000247.
Zaichenko, M. I., Bazhenova, D. A., Grigoryan, G. A., and Merzhanova, G. Kh., “Does the property of impulsivity affect the manifestations of long-term and short-term memory in rats?” Zh. Vyssh. Nerv. Deyat., 66, No. 1, 82–91 (2016b).
Zaichenko, M. I., Grigoryan, G. A., and Merzhanova, G. Kh., “Escape and avoidance reactions of electrocutaneous stimulation of the self and other rats with high and low levels of impulsivity,” Zh. Vyssh. Nerv. Deyat., 68, No. 4, 477–487 (2018).
Zaichenko, M. I., Sharkova, A. V., Grigoryan, G. A., and Merzhanova, G. Kh., “Impulsivity improves cue memory in an eight-channel maze in rats,” Zh. Vyssh. Nerv. Deyat., 66, No. 5, 600–610 (2016a).
Zoratto, F., Laviola, G., and Adriani, W., “The subjective value of probabilistic outcomes: Impact of reward magnitude on choice with uncertain rewards in rats,” Neurosci. Lett., 617, 225–231 (2016).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Zhurnal Vysshei Nervnoi Deyatel’nosti imeni I. P. Pavlova, Vol. 70, No. 2, pp. 231–242, March–April, 2020.
Rights and permissions
About this article
Cite this article
Zaichenko, M.I., Merzhanova, G.K. & Grigoryan, G.A. Ability to Discriminate Visual Signals in the Morris Water Maze in High- and Low-Impulsivity Rats. Neurosci Behav Physi 50, 1155–1162 (2020). https://doi.org/10.1007/s11055-020-01018-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11055-020-01018-6