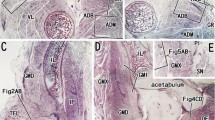
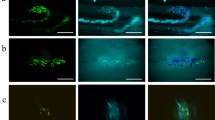
The gastrocnemius muscle in white mongrel female rats was studied from postnatal day 14 to postnatal day 180 in normal conditions (control group, n = 18), after neonatal chemical deafferentation (n = 18), after neonatal chemical desympathization (n = 18), and after combined neonatal chemical denervation (deafferentation plus desympathization, n = 18). Controls consisted of intact animals (n = 18). Histochemical methods were used to detect cholinesterase (using thioacetic acid as substrate) and alkaline phosphatase (using naphthol AS-BS as substrate) activities. Combined chemical denervation showed a reduction in cholinesterase activity compared with controls, block of early developmental elimination of neuromuscular synapses, a lower proportion of complex synapse-active zones, and later formation of vascular loops around motor endings. The vascular network had larger-diameter vessels, most with a planar orientation. The forming “muscle fi ber – motor ending – vascular network” system had low adaptive potential and a tendency to earlier development of involutional processes.
Similar content being viewed by others
References
V. A. Govyrin, “Adaptive-trophic functions of nerve vessels,” in: Development of the Scientific Legacy of Academician L. A. Orbeli, Nauka, Leningrad (1982), pp. 169–181.
A. S. Golub’ and V. I. Brod, “Developmental changes in measures of capillary blood fl ow in skeletal muscle: allometric relationships and the capillary functional length hypothesis,” Ros. Fiziol. Zh., 79, No. 4, 43–52 (1993).
T. R. Kovrigina and V. I. Filimonov, “Differentiation of the skeletal muscle of the shins during postnatal ontogeny,” Morfologiya, 137, No. 3, 36–40 (2010).
T. R. Kovrigina and V. I. Filimonov, “Characteristics of the microcirculatory bed of the gastrocnemius muscle in deafferented white rats,” Uch. Zapiski St.-Peterb. Gos. Med. Univ., 18, No. 2, 67–69 (2011).
T. R. Kovrigina and V. I. Filimonov, “Reactions of the microcirculatory bed of skeletal muscle to deficit of sympathetic innervation,” Astrakhan. Med. Zh., 8, No. 1, 131–133 (2013).
V. I. Kozlov, E. P. Mel’man, E. M. Neiko, and B. V. Shutka, The Histophysiology of Capillaries, Nauka, St. Petersburg (1994).
A. F. Nikiforov, Afferent Neurons and Neurodystrophic Changes, Meditsina, Moscow (1973).
G. M. Nikolaev and V. V. Shilkin, “Experience in assessing the acetylcholinesterase activity in peripheral nervous system structures,” in: Problems in the Morphogenesis of Peripheral Nerves: Collection of Studies at the Yaroslavl Medical Institute, Yaroslavl (1983), pp. 64–72.
E. Pearse, Histochemistry [Russian translation], Izd. Inostr. Lit., Moscow (1962).
I. M. Rodionov, V. N. Yarygin, and A. A. Mukhammedov, Immu nological and Chemical Desympathization, Nauka, Moscow (1988).
T. A. Rumyantseva, Effects of Chemical Denervation on Neurocytes in Extra- and Intramural Ganglia during Postnatal Ontogeny in the White Rat: Auth. Abstr. Dissert. Doct. Med. Sci., St. Petersburg (2002).
V. M. Chuchkov, G. P. Kotel’nikov, and P. A. Gelashvili, “Systematic multifactorial analysis of reactive rearrangements of microcirculatory modules in different types of skeletal muscles in mammals in conditions of impaired blood fl ow,” Morfol. Vedom., No. 3, 4, 68–70 (2004).
J. A. Buttner-Ennever, A. Eberhorn, and A. K. Horn, “Motor and sensory innervation of extraocular eye muscles,” Ann. N.Y. Acad. Sci., 1004, 40–49 (2003).
A. Hirua, “Neuroanatomical effects of capsaicin on the primary afferent neurons,” Arch. Histol. Cytol., 63, No. 3, 199–215 (2000).
H. S. Neto and M. J. Marques, “Microvessel damage by Bothropus jararacussu snake venom: pathogenesis and influence on muscle regeneration,” Toxicon, 46, No. 7, 814–819 (2005).
E. Ralston, Z. Lu, N. Biscocho, et al., “Blood vessels and desmin control the positioning of nuclei in skeletal muscle fibers,” J. Cell. Physiol., 209, No. 3, 874–882 (2006).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Translated from Morfologiya, Vol. 146, No. 6, pp. 54–59, November–December, 2014.
Rights and permissions
About this article
Cite this article
Kovrigina, T.R., Filimonov, V.I. The Vascular Network and Neuromuscular Synapses in Skeletal Muscle in Combined Chemical Denervation. Neurosci Behav Physi 45, 996–1000 (2015). https://doi.org/10.1007/s11055-015-0177-6
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11055-015-0177-6