Abstract
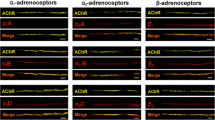
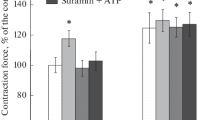
Influence of the sympathetic nervous system on the work of skeletal muscles contractile apparatus is now beyond doubt. However, until recently there was no evidence that the endings of sympathetic nerves can be located in close proximity to the neuromuscular synapses, and there is also no reliable data on how much endogenous adrenaline and noradrenaline can be contained near the synaptic contact in skeletal muscles. In this research, using fluorescent analysis, immunohistochemical and enzyme immunoassays the isolated neuromuscular preparations of three skeletal muscles of different functional profiles and containing different types of muscle fibers were examined. Close contact between the sympathetic and motor cholinergic nerve endings and the presence of tyrosine hydroxylase in this area were demonstrated. Concentrations of endogenous adrenaline and noradrenaline in the solution perfusing the neuromuscular preparation were determined under different modes of its functioning. The effects of α and β adrenoreceptor blockers on the processes of acetylcholine quantal secretion from the motor nerve endings were compared. The data obtained provide evidence for the presence of endogenous catecholamines in the neuromuscular junction region and their role in modulation of the synaptic function.



Similar content being viewed by others
Abbreviations
- AD:
-
adrenaline
- ADR:
-
adrenoreceptors
- NA:
-
noradrenaline
- NMP:
-
neuromuscular preparation
References
Corkill, A. B., and Tiegs, O. W. (1933) The effect of sympathetic nerve stimulation on the power of contraction of skeletal muscle, J. Physiol., 78, 161-185, https://doi.org/10.1113/jphysiol.1933.sp002995.
Brown, G. L., Bülbring, E., and Burns, B. D. (1948) The action of adrenaline on mammalian skeletal muscle, J. Physiol., 107, 115-128, https://doi.org/10.1113/jphysiol.1948.sp004255.
Steiner, J. L., Johnson, B. R., Hickner, R. C., Ormsbee, M. J., Williamson, D. L., and Gordon, B. S. (2021) Adrenal stress hormone action in skeletal muscle during exercise training: An old dog with new tricks?, Acta Physiol. (Oxf), 231, e13522, https://doi.org/10.1111/apha.13522.
Kvetnansky, R., Lu, X., and Ziegler, M. G. (2013) Stress-triggered changes in peripheral catecholaminergic systems, Adv. Pharmacol., 68, 359-397, https://doi.org/10.1016/B978-0-12-411512-5.00017-8.
Tank, A. W., and Lee Wong, D. (2015) Peripheral and central effects of circulating catecholamines, Compr. Physiol., 5, 1-15, https://doi.org/10.1002/cphy.c140007.
Zouhal, H., Jacob, C., Delamarche, P., and Gratas-Delamarche, A. (2008) Catecholamines and the effects of exercise, training and gender, Sports Med., 38, 401-423, https://doi.org/10.2165/00007256-200838050-00004.
Andersson, D. C., Betzenhauser, M. J., Reiken, S., Umanskaya, A., Shiomi, T., and Marks, A. R. (2012) Stress-induced increase in skeletal muscle force requires protein kinase A phosphorylation of the ryanodine receptor, J. Physiol., 590, 6381-6387, https://doi.org/10.1113/jphysiol.2012.237925.
Khan, M. M., Lustrino, D., Silveira, W. A., Wild, F., Straka, T., Issop, Y., O'Connor, E., Cox, D., Reischl, M., Marquardt, T., Labeit, D., Labeit, S., Benoit, E., Molgó, J., Lochmüller, H., Witzemann, V., Kettelhut, I. C., Navegantes, L. C., Pozzan, T., and Rudolf, R. (2016) Sympathetic innervation controls homeostasis of neuromuscular junctions in health and disease, Proc. Natl. Acad. Sci. USA, 113, 746-750, https://doi.org/10.1073/pnas.1524272113.
Straka, T., Vita, V., Prokshi, K., Hörner, S. J., Khan, M. M., Pirazzini, M., Williams, M. P. I., Hafner, M., Zaglia, T., and Rudolf, R. (2018) Postnatal development and distribution of sympathetic innervation in mouse skeletal muscle, Int. J. Mol. Sci., 19, 1935, https://doi.org/10.3390/ijms19071935.
Kim, J., Grotegut, C. A., Wisler, J. W., Li, T., Mao, L., Chen, M., Chen, W., Rosenberg, P. B., Rockman, H. A., and Lefkowitz, R. J. (2018) β-arrestin 1 regulates β2-adrenergic receptor-mediated skeletal muscle hypertrophy and contractility, Skelet. Muscle, 8, 39, https://doi.org/10.1186/s13395-018-0184-8.
Williams, R. S., Caron, M. G., and Daniel, K. (1984) Skeletal muscle beta-adrenergic receptors: variations due to fiber type and training, Am. J. Physiol., 246, 160-167, https://doi.org/10.1152/ajpendo.1984.246.2.E160.
Hinkle, R. T., Hodge, K. M. B., Cody, D. B., Sheldon, R. J., Kobilka, B. K., and Isfort, R. J. (2002) Skeletal muscle hypertrophy and anti-atrophy effects of clenbuterol are mediated by the beta2-adrenergic receptor, Muscle Nerve, 25, 729-734, https://doi.org/10.1002/mus.10092.
Lynch, G. S., and Ryall, J. G. (2008) Role of beta-adrenoceptor signaling in skeletal muscle: implications for muscle wasting and disease, Physiol. Rev., 88, 729-767, https://doi.org/10.1152/physrev.00028.2007.
Tsentsevitsky, A., Kovyazina, I., and Bukharaeva, E. (2019) Diverse effects of noradrenaline and adrenaline on the quantal secretion of acetylcholine at the mouse neuromuscular junction, Neuroscience, 423, 162-171, https://doi.org/10.1016/j.neuroscience.2019.10.049.
Nagatsu, T., Levitt, M., and Udenfriend, S. (1964) Tyrosine hydroxylase. The initial step in norepinephrine biosynthesis, J. Biol. Chem., 239, 2910-2917, https://doi.org/10.1016/S0021-9258(18)93832-9.
Tsentsevitsky, A., Nurullin, L., Tyapkina, O., and Bukharaeva, E. (2020) Sympathomimetics regulate quantal acetylcholine release at neuromuscular junctions through various types of adrenoreceptors, Mol. Cell. Neurosci., 108, 103550, https://doi.org/10.1016/j.mcn.2020.103550.
Arkhipov, A., Khuzakhmetova, V., Petrov, A. M., and Bukharaeva, E. A. (2022) Catecholamine-dependent hyperpolarization of the junctional membrane via β2-adrenoreceptor/Gi-protein/α2-Na-K-ATPase pathway, Brain Res., 1795, 148072, https://doi.org/10.1016/j.brainres.2022.148072.
Khuzakhmetova, V., and Bukharaeva, E. (2020) Adrenaline facilitates synaptic transmission by synchronizing release of acetylcholine quanta from motor nerve endings, Cell. Mol. Neurobiol., 41, 395-401, https://doi.org/10.1007/s10571-020-00840-3.
Anderson, A. J., and Harvey, A. L. (1988) Effects of the facilitatory compounds catechol, guanidine, noradrenaline and phencyclidine on presynaptic currents of mouse motor nerve terminals, Naunyn. Schmiedebergs. Arch. Pharmacol., 338, 133-137, https://doi.org/10.1007/bf00174860.
Bukcharaeva, E. A., Kim, K. C., Moravec, J., Nikolsky, E. E., and Vyskočil, F. (1999) Noradrenaline synchronizes evoked quantal release at frog neuromuscular junctions, J. Physiol., 517, 879-888, https://doi.org/10.1111/j.1469-7793.1999.0879s.x.
Kuba, K. (1970) Effects of catecholamines on the neuromuscular junction in the rat diaphragm, J. Physiol., 211, 551-570, https://doi.org/10.1113/jphysiol.1970.sp009293.
Kuba, K., and Tomita, T. (1971) Noradrenaline action on nerve terminal in the rat diaphragm, J. Physiol., 217, 19-31, https://doi.org/10.1113/jphysiol.1971.sp009557.
Rodrigues, A. Z., Wang, Z. M., Messi, M. L., and Delbono, O. (2019) Sympathomimetics regulate neuromuscular junction transmission through TRPV1, P/Q- and N-type Ca2+ channels, Mol. Cell. Neurosci., 95, 59-70, https://doi.org/10.1016/j.mcn.2019.01.007.
Gubernator, N. G., Zhang, H., Staal, R. G., Mosharov, E. V., Pereira, D. B., Yue, M., Balsanek, V., Vadola, P. A., Mukherjee, B., Edwards, R. H., Sulzer, D., and Sames, D. (2009) Fluorescent false neurotransmitters visualize dopamine release from individual presynaptic terminals, Science, 324, 1441-1444, https://doi.org/10.1126/science.1172278.
Betz, W. J., and Bewick, G. S. (1992) Optical analysis of synaptic vesicle recycling at the frog neuromuscular junction, Science, 255, 200-203, https://doi.org/10.1126/science.1553547.
Reid, B., Slater, C. R., Bewick, G. S. (1999) Synaptic vesicle dynamics in rat fast and slow motor nerve terminals, J. Neurosci., 19, 2511-2521, https://doi.org/10.1523/JNEUROSCI.19-07-02511.1999.
Petrov, A., Zakirjanova, G., Kovyazina, I., Tsentsevitsky, A., and Bukharaeva, E. (2022) Adrenergic receptors control frequency-dependent switching of the exocytosis mode between “full-collapse” and “kiss-and-run” in murine motor nerve terminal, Life Sci., 296, 120433, https://doi.org/10.1016/j.lfs.2022.120433.
Murphy, J. F., Davies, D. H., and Smith, C. J. (1992) The development of enzyme-linked immunosorbent assays (ELISA) for the catecholamines adrenalin and noradrenalin, J. Immunol. Meth., 154, 89-98, https://doi.org/10.1016/0022-1759(92)90216-g.
Slater, C. R. (2015) The functional organization of motor nerve terminals, Prog Neurobiol., 134, 55-103, https://doi.org/10.1016/j.pneurobio.2015.09.004.
Westermann, J., Hubl, W., Kaiser, N., and Salewski, L. (2002) Simple, rapid and sensitive determination of epinephrine and norepinephrine in urine and plasma by non-competitive enzyme immunoassay, compared with HPLC method, Clin Lab., 48, 61-71.
Tekin, I., Roskoski, R. Jr., Carkaci-Salli, N., and Vrana, K. E. (2014) Complex molecular regulation of tyrosine hydroxylase, J. Neural. Transm., 121, 1451-1481, https://doi.org/10.1007/s00702-014-1238-7.
Bruhwyler, J., Liégeois, J. F., and Géczy, J. (1997) Pirlindole: a selective reversible inhibitor of monoamine oxidase A. A review of its preclinical properties, Pharmacol. Res., 36, 23-33, https://doi.org/10.1006/phrs.1997.0196.
Peyrin, L., and Dalmaz, Y. (1975) Peripheral secretion and inactivation of catecholamines (adrenaline, noradrenaline, dopamine) [In French], J. Physiol., 70, 353-433.
Eisenhofer, G., Kopin, I., and Goldstein, D. (2004) Catecholamine metabolism: a contemporary view with implications for physiology and medicine, Pharmacol. Rev., 56, 331-349, https://doi.org/10.1124/pr.56.3.1.
Hjemdahl, P., Daleskog, M., and Kahan, T. (1979) Determination of plasma catecholamines by high performance liquid chromatography with electrochemical detection: comparison with a radioenzymatic method, Life Sci., 25, 131-138, https://doi.org/10.1016/0024-3205(79)90384-9.
Liao, Y. T., Wang, S. M., Wang, J. R., Yu, C. K., and Liu, C. C. (2015) Norepinephrine and epinephrine enhanced the infectivity of enterovirus, PLoS One, 10, e0135154, https://doi.org/10.1371/journal.pone.0135154.
Li, J., King, N. C., and Sinoway, L. I. (2005) Interstitial ATP and norepinephrine concentrations in active muscle, Circulation, 111, 2748-2751, https://doi.org/10.1161/CIRCULATIONAHA.104.510669.
Martin, W. H., Murphree, S. S., and Saffitz, J. E. (1989) Beta-adrenergic receptor distribution among muscle fiber types and resistance arterioles of white, red, and intermediate skeletal muscle, Circ. Res., 64, 1096-1105, https://doi.org/10.1161/01.res.64.6.1096.
Dorszewska, J., Prendecki, M., Oczkowska, A., Rozycka, A., Lianeri, M., and Kozubski, W. (2013) Polymorphism of the COMT, MAO, DAT, NET and 5-HTT genes, and biogenic amines in Parkinson’s disease, Curr. Genomics, 14, 518-533, https://doi.org/10.2174/1389202914666131210210241.
Goldstein, D. S. (2020) The “sick-but-not-dead” phenomenon applied to catecholamine deficiency in neurodegenerative diseases, Semin. Neurol., 40, 502-514, https://doi.org/10.1055/s-0040-1713874.
Abercrombie, E. D., Keller, R. W. Jr, and Zigmond, M. J. (1988) Characterization of hippocampal norepinephrine release as measured by microdialysis perfusion: pharmacological and behavioral studies, Neuroscience, 27, 897-904, https://doi.org/10.1016/0306-4522(88)90192-3.
Lim, S. P., and Muir, T. C. (1983) Microelectrode recording of the effects of agonists and antagonists on alpha-adrenoceptors on rat somatic nerve terminals, Br. J. Pharmacol., 80, 41-46, https://doi.org/10.1111/j.1476-5381.1983.tb11047.x.
Wang, Z. M., Rodrigues, A. C. Z., Messi, M. L., and Delbono, O. (2020) Aging blunts sympathetic neuron regulation of motoneurons synaptic vesicle release mediated by β1- and α2B-adrenergic receptors in geriatric mice, J. Gerontol. A Biol. Sci. Med. Sci., 75, 1473-1480, https://doi.org/10.1093/gerona/glaa022.
Rodrigues, A. C. Z., Messi, M. L., Wang, Z. M., Abba, M. C., Pereyra, A., Birbrair, A., Zhang, T., O’Meara, M., Kwan, P., and Lopez, E. I. S. (2019) The sympathetic nervous system regulates and acetylcholine receptor stability, Acta Physiol., 225, 13195, https://doi.org/10.1111/apha.13195.
Acknowledgments
The authors express their gratitude to A.M. Petrov for critical review of the manuscript and helpful suggestions.
Funding
This work was financially supported by the Russian Science Foundation (project no. 18-15-0046). ELISA, sample preparation and data analysis were performed by S.A. Dmitrieva and S.G. Vologin with the support from the government assignment for FRC Kazan Scientific Center of the Russian Academy of Sciences.
Author information
Authors and Affiliations
Contributions
E.A. Bukharaeva – conceptualization, supervision, methodology, writing, review & editing; S.A. Dmitrieva, S.G. Vologin – enzyme immunoassay; A.N. Tsentsevitzky, A.Yu. Arkhipov, V.F. Khuzakhmetova – preparing and investigation of experimental samples; G.V. Sibgatullina – immunohistochemical analysis.
Corresponding author
Ethics declarations
The authors declare no conflict of interest in financial or any other sphere. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Rights and permissions
About this article
Cite this article
Dmitrieva, S.A., Vologin, S.G., Tsentsevitsky, A.N. et al. Sympathetic Innervation and Endogenous Catecholamines in Neuromuscular Preparations of Muscles with Different Functional Profiles. Biochemistry Moscow 88, 364–373 (2023). https://doi.org/10.1134/S0006297923030069
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0006297923030069