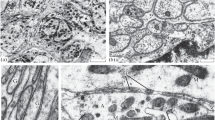
The aim of the present work was to study the contractile activity of traumatized nerve cell processes and to attempt to inhibit their retraction using a solution of colchicine. Experiments were performed on living isolated neurons from freshwater mollusks (Lymnaea stagnalis and Planorbis corneus vulgaris), which were studied in phase contrast conditions using time-lapse microvideo recordings. Contractile activity of nerve cell processes was seen in 92% of cases in control conditions in Ringer’s solution. Colchicine inhibited nerve process contraction in 86% of neurons. Experiments addressing neuron electrical activity were performed on leech Retzius neurons. Incubation of ganglia in colchicine solution was found to increase the frequency of spontaneous spike activity from 0.22 to 0.75 spikes/sec. The amplitude of spontaneous potentials decreased from 46.9 to 37 mV, the threshold decreased by 18%, the duration of spontaneous spikes increased from 4.3 to 7.1 msec, and the latent period of responses to stimuli increased from 25.0 to 37.9 msec. In conditions of stimulation at 7–10 Hz, neurons generated spike activity at higher frequencies than in control conditions. Thus, our experiments showed that colchicine can inhibit the contractile activity of traumatized nerve cell processes, keeping the electrically excitable membrane in a satisfactory condition. It follows that attempts can be made in vivo to produce partial inhibition of nerve fiber contraction, thus preventing increases in neuron diastasis, which prevent surgical approximation to the point of contact and promote the development of massive scars at transection sites.
Similar content being viewed by others
References
A. Alberts, D. Bray, R. Lewis, et al., Molecular Biology of the Cell [Russian translation], Mir, Moscow (19904), Vol. 3.
Yu. M. Vasil’ev, “The cell as an architectural wonder,” Sorosov. Obrazovat. Zh., No. 2, 9–11 (1996).
N. Yu. Vasyagina, S. S. Sergeeva, O. S. Sotnikov, et al., “Effects of cytochalasin B on the contractive activity of damaged nerves,” Tsitologiya, 54, No. 9, 671–672 (2012).
N. N. Kamiya, Protoplasmic Streaming [Russian translation], Mir, Moscow (1962).
Kh. Koshtoyants, Basic Comparative Physiology [in Russian], Vol. II, Comparative Physiology of the Nervous System [in Russian], USSR Academy of Sciences Press, Moscow (1957).
S. S. Sergeeva, “Electrophysiological studies of the topography of axodendritic synapses of Retzius neurons in leeches,” Ros. Fiziol. Zh., 84, No. 10, 117–120 (1995).
O. S. Sotnikov, N. Yu. Vasyagin, G. I. Rybakova, and S. V. Chepur, “Attempts to inhibit nerve process contraction in medium lacking calcium ions,” Byull. Eksperim. Biol., 149, No. 2, 232–235 (2010).
T. A. Uzbekova, I. A. Chernova, V. I. Savchuk, et al., “Methods of applying colchicine to the rat vagus nerve using selective action of neuron axonal transport,” Byull. Eksperim. Biol., 92, No. 11, 631–634 (1981).
C. E. Aguilar, M. A. Bisby, E. Cooper, et al., “Evidence that transport of trophic factors is involved in the regulation of peripheral nerve fields in salamanders,” J. Physiol., 234, No. 2, 449–464 (1973).
R. Bai, X. F. Pei, O. Boyé, et al., “Identification of cysteine 354 of beta-tubulin as part of the binding site for the A ring of colchicine,” Biol. Chem., 271, No. 21, 12639–12645 (1996).
A. Bouron, “Colchicine affects protein kinase C-induced modulation of synaptic transmission in cultured hippocampal pyramidal cells,” FEBS Lett., 404, No. 2–3, 221–226. (1997).
K. Bracey, M. Ju, C. Tian, et al., “Tubulin as a binding partner of the heag2 voltage-gated potassium channel,” J. Membr. Biol., 222, No. 3, 115–125 (2008).
P. Coulon, H. J. Wüsten, P. Hochstrate, et al., “Swelling-activated chloride channels in leech Retzius neurons,” J. Exp. Biol., 211, No. 4, 630–641 (2008).
J. Gordiner, R. Overall, and J. Marc, “The microtubule cytoskeleton acts as a key downstream effector of neurotransmitter signaling,” Synapse, 65, 249–256 (2011).
Y. He, W. Yu, and P. W. Baas, “Microtubule reconfiguration during axonal retraction induced by nitric oxide,” J. Neurosci., 22, 5982–5991 (2002).
S. T. Hou, S. X. Jiang, and R. A. Smith, “Permissive and repulsive cues and signaling pathways of axonal outgrowth and regeneration,” Jnt. Rev. Cell Mol. Biol., 267, 125–181 (2008).
S. H. Huang,Y. I. Wang, G. F. Tseng, et al., “Active endocytosis and microtubule remodeling restore compressed pyramidal neuron morphology in rat cerebral cortex,” Cell Mol. Neurobiol., 32, No. 7, 1079–1087 (2012).
J. J. Li, S. H. Lee, D. K. Kim, et al., “Colchicine attenuates inflammatory cell infiltration and extracellular matrix accumulation in diabetic neuropathy,” Am. J. Physiol. Renal Physiol., 297, No. 1, 200–209 (2009).
L. Luo and D. D. M. O’Leary, “Axon retraction and degeneration in development and disease,” Annu. Rev. Neurosci., 28, 127–156 (2005).
G. Matsumoto, “A proposed membrane model for generation of sodium currents in quid giant axons,” J. Theor. Biol., 107, 649–666 (1984).
S. L. Mironov and D. W. Richter, “Cytoskeleton mediates inhibition of the fast Na+ current in respiratory brainstem neurons during hypoxia,” Eur. J. Neurosci., 11, No. 5, 1831–1834 (1999).
D. T. Moran and F. G. Varela, “Microtubules and sensory transduction,” Proc. Natl. Acad. Sci. USA, 68, No. 4, 757–760 (1971).
C. Sayas, A. Ariaens, B. Ponsioen, et al., “GSK-3 is activated by the tyrosine kinase Pyk2 during LPA1-mediated neurite retraction,” Mol. Biol. Cell, 17, No. 4, 1834–18944 (2006).
R. Schafer and P. D. Reagan, “Colchicine reversibly inhibits electrical activity in arthropod mechanoreceptors,” J. Neurobiol., 12, No. 2, 155–166 (1981).
Y. Solak, H. Atalay, I. Polat, et al., “Colchicine treatment in autosomal dominant polycystic kidney disease: many points in common,” Med. Hypoth., 74, No. 2, 314–317 (2010).
C. C. Speidel, “The experimental induction of visible structural changes in single nerve fibers in living frog tadpoles,” Cold Spring. Harbor Symp. Quant. Biol., 4, 13–17 (1936).
A. Tandon, M. Bachoo, P. Weldon, et al., “Effect of colchicine application to preganglionic axons on choline acetyltransferase activity and acetylcholine content and release in the superior cervical ganglion,” J. Neurochem., 66, No. 3, 1033–1041.
R. I. Watts, E. D. Hoopfer, and L. Luo, “Axon pruning during Drosophila metamorphosis. Evidence for local degeneration and requirement of the ubiquitin-proteasome system,” Neuron, 38, 871–885 (2003).
L. A. White, P. W. Baas, and S. R. Heidemann, “Microtubule stability in severed axons,” J. Neurocytol., 16, 775–784 (1987).
R. Wong and I. W. Lichtman, “Synapse elimination,” in: Fundamentals of Neuroscience, Academic Press, San Diego (2003)., pp. 533–554.
K. M. Yamada, B. S. Spooner, H. K. Wessells, et al., “Microfilaments and microtubules,” Proc. Natl. Acad. Sci. USA, 66, 1206–1212 (1970).
X-F. Zhang, A. W. Schaefer, D. T. Bernette, et al., “Rho-dependent contractile responses in the neuronal growth cone are independent of classical peripheral retrograde actin flow,” Neuron, 40, No. 5, 931–944 (2003).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Morfologiya, Vol. 142, No. 6, pp. 25–29, November–December, 2012.
Rights and permissions
About this article
Cite this article
Sergeeva, S.S., Vasyagina, N.Y., Sotnikov, O.S. et al. Contractile and Electrical Activity of Neurons on Exposure to Colchicine. Neurosci Behav Physi 43, 1092–1096 (2013). https://doi.org/10.1007/s11055-013-9854-5
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11055-013-9854-5