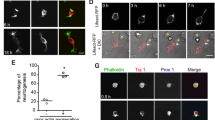
The classical Bielschowsky–Gross neurohistological method was used to reproduce all the morphological phenomena interpreted by many authors as signs of neuron division, budding, and fission. It is suggested that these signs are associated with the effects of enucleation, which occurs in many cells of other tissue types in response to a variety of chemical and physical treatments. Studies were performed using neurons isolated from the mollusk Lymnaea stagnalis and exposed in tissue culture to the actin microfilament inhibitor cytochalasin B. Phase contrast time-lapse video recording over periods of 4–8 h demonstrated nuclear displacement, ectopization, and budding, to the level of almost complete fission of the neuron body. This repeats the pattern seen in static fixed preparations in “normal” conditions and after different experimental treatments. Budding of the cytoplasm was also sometimes seen at the early stages of the experiments. Control experiments in which cultured neurons were exposed to the solvent for cytochalasin B, i.e., dimethylsulfoxide (DMSO), did not reveal any changes in neurons over a period of 8 h. We take the view that the picture previously interpreted as neuron division and fission can be explained in terms of the inhibition of actin microfilaments, sometimes developing spontaneously in cells undergoing individual metabolic changes preventing the maintenance of cytoskeleton stability.
Similar content being viewed by others
References
A. S. Altshul, “Changes in neural ganglia in the digestive tract in experimental intestinal obstruction,” Arkh. Biol. Nauk, 58, No. 1, 124–129 (1940).
M. V. Voino-Yasenetskii, and Yu. M. Zhabotinskii, Sources of Errors in Morphological Studies [in Russian], Meditsina, Leningrad (1970).
B. A. Dolgo-Saburov, The Innervation of Veins [in Russian], Medgiz, Leningrad (1958).
E. E. Egorov, I. A. Prudovskii, and A. V. Selenin, “Comparative studies of L-cell cytoplasts prepared using and not using cytochalasin B,” Dokl. Akad. Nauk SSSR, 264, No. 4, 969–973 (1982).
Yu. M. Zhabotinskii, Normal and Pathological Morphology of Autonomic Ganglia [in Russian], Academy of Medical Sciences of the USSR Press (1953).
Yu. M. Zhabotinskii, Normal and Pathological Morphology of Neurons [in Russian], Meditsina, Leningrad (1965).
A. V. Zelenin, A. A. Kushch, and I. A. Produvskii, Reconstruction of Cells [in Russian], Nauka, Moscow (1982).
O. Yu. Ivanova, E. A. Smirnova, and S. G. Komi, “Mechanisms of formation of multinucleate cells in the presence of cytochalasin B in cultures of transformed fibroblasts,” Tsitologiya, 27, No. 7, 780–784 (1985).
G. A. Koblov, Nerve Cell Division, [in Russian], Saratov University Press, Saratov (1974).
A. A. Laktionova and O. S. Sotnikov, “Studies of the phenomenon of ‘neuron division’ in living cells,” Morfologiya, 136, No. 4, 87 (2009).
F. Lominskii, “Experimental studies in adult animals and embryos – can nerve cells multiply by division?” Universitetskie Izvestiya, No. 3, 1039 (Addendum) (1882).
K. M. Morozova and E. V. Kiseleva, “Changes in the organization of the nucleus and cytoplasm of Xenopus oocytes after degradation of actin filaments with latrunculine,” Tsitologiya, 50, No. 5, 394–405 (2008).
I. A. Prudovskii, A. Yu. Kerkis, S. I. Baiborodin, et al., “Use of cell enucleation to study the stability of cytoplasmic organelles and the organization of the cytoplasm,” Tsitologiya, 27, No. 7, 792–795 (1985).
T. N. Radostina, “The multiplication of autonomic nervous system neurons,” in: The Influences of the Higher Centers of the Nervous System on Inflammation and Regeneration Processes. Studies at the Moscow Medical Institute [in Russian] (1957), pp. 241–249.
N. Ringertz and R. Savage, Hybrid Cells [Russian translation], Mir, Moscow (1979).
V. V. Serov and V. S. Paukov, Ultrastructural Pathology [in Russian], Meditsina, Moscow (1975).
N. E. Yarygin and V. N. Yarygin, Pathological and Adaptive Changes in Neurons [in Russian], Meditsina, Moscow (1973).
M. T. Bohnsack, T. Stuven, C. Kuhn, et al., “A selective block of nuclear actin export stabilizes the giant nuclei of Xenopus oocytes,” Nat. Cell. Biol., 8, 257–263 (2006).
S. B. Carter, “Effects of cytochalasins on mammalian cells,” Nature, 213, 261–266 (1967).
N. Chen, S. L. Liow, W. Y. Yip, et al., “Early development of reconstructed embryos after somatic cell nuclear transfer in a non-human primate,” Theriogenology, 66, No. 5, 1300–1306 (2006).
V. C. Coimbra, D. Yamamoto, K. G. Khusal, et al., “Enucleated L929 cells support invasion, differentiation, and multiplication of Trypanosoma cruzi parasites,” Infect. Immun., 75, No. 8, 3700–3706 (2007).
K. Hosaka, S. Ohi, A. Ando, et al., “Cloned mice derived from somatic cell nuclei,” Hum. Cell, 13, No. 4, 237–242 (2000).
T. Iwai, “Temporal profile of neural stem cell proliferation in the sub-ventricular zone after ischemia/hypoxia in the neonatal rat brain,” Neurol. Res., 28, No. 4, 461–468 (2006).
M. Kawahara, T. Mori, H. Tanaka, and H. Shimizu, “The suppression of fragmentation by stabilization of actin filament in porcine enucleated oocytes,” Theriogerontology, 58, No. 6, 1081–1095 (2002).
G. C. Lan, Y. C. Wu, D. Han, et al., “Demecolcine – assisted enucleation of goat oocytes: protocol optimization, mechanism investigation, and application to improve the developmental potential of cloned embryos,” Cloning Stem Cells, 10, No. 2, 189–202 (2008).
D. Liberman and L. Sachs, “Nuclear control of neurite induction in neuroblastoma cells,” Exp. Cell Res., 113, No. 2, 383–390 (1978).
C. Mirescu, J. D. Peters, and E. Gould, “Early life experience alters response of adult neurogenesis to stress,” Nat. Neurosci., 7, No. 8, 841–846 (2004).
R. A. Nichols, C. E. Chandler, and E. M. Shooter, “Enucleation of the rat pheochromocytoma clonal cell line, PC 12: effect on neurite outgrowth,” J. Cell Physiol., 141, No. 2, 301–309 (1989).
F. Nissl, “Über die Veränderungen der Ganglienzellen am Fascialisnerv der Kaninchen nach Ausreissung der Nerven,” Allg. Zschr. Psych., 48, No. 197, 675–689 (1892).
S. Ramon y Cajal, Degeneration and Regeneration of the Nervous System, Hafner Publishing Co., New York (1959).
E. A. Repasky and B. S. Eckert, “The effect of cytochalasins B on the enucleation of erythroid cells in vitro,” Cell Tiss. Res., 221, No. 1, 85–91 (1981).
J. W. Shay, K. R. Porter, and D. M. Prescott, “The surface morphology and fine structure of CHO (Chinese hamster ovary) cells following enucleation,” Proc. Natl. Acad. Sci. USA, 71, No. 8, 3059–3063 (1974).
J. Tesarik, F. Martinez, L. Rienzi, et al., “Microfilament disruption is required for enucleation and nuclear transfer in germinal vesicle but not meta phase II human oocytes,” Fertil. Steril., 79, Supplement 1, 677–681 (2003).
V. Volloch, B. Schweitzer, and S. Rits, “Synthesis of globin RNA in enucleated differentiating murine erythroleukemia cells,” J. Cell Biol., 105, No. 1, 137–143 (1987).
D. L. Yamamoto, V. C. Coimbra, K. Okuda, and M. Rabinovitch, “Enucleated L929 mouse fibroblasts support invasion and multiplication of Shigella flexneri 5a,” Braz. J. Med. Biol. Res., 39, No. 6, 749–758 (2006).
Author information
Authors and Affiliations
Additional information
Translated from Morfologiya, Vol. 136, No. 6, pp. 28–34, November–December, 2009. Original article submitted April 27, 2009.
Rights and permissions
About this article
Cite this article
Sotnikov, O.S., Laktionova, A.A., Solovieva, I.A. et al. Neuron Division or Enucleation. Neurosci Behav Physi 40, 841–847 (2010). https://doi.org/10.1007/s11055-010-9339-8
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11055-010-9339-8