Abstract
Glioblastoma (GBM), the most prevalent primary brain tumor in adults, remains highly challenging due to its invasive nature, limited treatment effectiveness, and short median survival durations. Standard of care includes surgery, radiation, chemotherapy, and tumor treating fields; however, there has been little improvement in survival rates. Biomimetic nanoparticles (NPs), coated with cell membranes and endogenous components, have immense potential for improving chemotherapy in GBM, by imitating cellular architecture and eluding immune clearance. With more individualized and efficient drug delivery, immunotherapeutic approaches and biomimetic NPs may increase patient survival rates. This article summarizes the main research on biomimetic NPs for GBM therapy, focusing on the classification, mechanisms, advantages, and challenges, along with the advancements in the development of GBM vaccines.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The World Health Organization (WHO) classifies glioblastoma (GBM) as a grade IV astrocytoma mostly located in the frontal, parietal, temporal, and occipital lobes, among other supratentorial brain regions which sometimes is seen in the cerebellum, brain stem, and spinal cord [1] and comprises up to 50% of all gliomas. Characterized by rapid, infiltrative growth, setting it apart from lower grade gliomas due to the presence of necrosis and/or microvascular proliferation, it may develop as a primary or secondary tumor, with the latter occurring due to a malignant transformation resulting from an inferior-grade brain tumor and/or a mutation in the isocitrate dehydrogenase (IDH) gene [2,3,4,5]. Ionizing radiation exposure stands out as a significant risk factor for GBM, alongside other risk factors such as age, gender, obesity, atopy history, allergies, and immune-related disorders. Rare genetic syndromes like Li–Fraumeni syndrome and Lynch syndrome account for less than 1% cases [6,7,8,9]. GBM patients that are newly diagnosed are usually treated with a multimodal approach, with surgery and concomitant radiotherapy and chemotherapy being the mainstay of treatment [10]. Nevertheless, surgery has its limitations as GBM is a highly diffuse, invasive, and vascularized tumor, risking the removal of healthy tissues; thus, treatments relied on alkylating drugs, primarily chloro-ethylating, nitrosourea derivatives such as carmustine, nimustine, and lomustine, which have demonstrated efficacy in GBM treatment through cellular death, but drawbacks include hepatic and pulmonary toxicity, as well as non-targeted distribution of chloro-ethylating agents, which may cause apoptosis in healthy cells [11]. Hence, the current standard of care post-surgery involves adjuvant and concurrent temozolomide (TMZ) and radiation therapy, often accompanied by using dexamethasone at large doses to treat vasogenic edema [12]. Nevertheless, chances are that postoperative radiation will result in radiation damage and a subsequent cancer in the exposed area [13]. Chemotherapy is another vital therapeutic strategy that can raise overall survival rates, but it has some systemic side effects [14]. Tumor treating fields (TTFs), a portable Optune device, apply an electric field to the tumor, representing one of the limited FDA-approved treatment options for newly diagnosed GBM [15]. A summary of the current therapies undergoing clinical trials is given in Table 1. Recurrence is inevitable, with most patients relapsing into a more aggressive tumor form [16,17,18]. Despite complete therapy, prognosis is poor, with a 5-year overall survival rate of 9.8%, a progression-free survival of 7–8 months, and a median survival of 14–16 months [3]. Symptoms from infiltrative tumor growth severely disrupt patients’ lives, gradually diminishing their quality of life [1, 3, 6, 16,17,18].
Challenges for drug delivery to the brain
The blood–brain barrier (BBB) is a semipermeable interface which regulates central nervous system homeostasis. It is composed of endothelial cells, tight junctions, receptors, transporters, and efflux pumps. The BBB acts as a structural barrier preventing most drugs including chemotherapeutic agents that are systemically delivered from reaching sufficient concentrations in the brain [14, 45, 46]. The distinct molecular features of GBM manifest a complex interplay of tumor heterogeneity, angiogenesis, and immunosuppressive mechanisms, collectively shaping its aggressive behavior and dismal prognosis [34]. Targeting these critical hallmarks of GBM—tumor heterogeneity, angiogenesis, and immunosuppression—is paramount in devising effective therapeutic strategies against this formidable brain cancer. GBM tumors comprise a diverse cell population, each exhibiting distinct molecular signatures and biological behaviors and a multiplicity of signaling pathways [35,36,37,38]. Meanwhile, angiogenesis, a hallmark feature of GBM, is sustained by overexpression of pro-angiogenic proteins such vascular endothelial growth factor (VEGF). This robust vascularization not only supports tumor growth but also facilitates the invasion of tumor cells into surrounding brain tissue. The poor immunogenicity of GBM prevents a robust immune response, and the TME increases the resistance of the tumor to radiation and chemotherapy [39]. Furthermore, the angiogenic propensities of GBM and the main obstacle to GBM treatment is temozolamide resistance via the O6MG methyltransferase (MGMT) [40,41,42,43,44]. Furthermore, the deep brain infiltration of glioma (stem) cells precludes therapeutic therapy with resection alone [1, 2].
Currently, there are no groundbreaking treatments that have resulted in substantial and lasting improvements in patient survival. To overcome the present obstacles in both conventional and experimental GBM treatment, the search for efficient drug delivery systems is ongoing [44].
The concept of using nanoparticles (NPs) to target cancerous tissues for better diagnosis and treatment has a longstanding presence [47, 48]. This is due to NP high drug payload, stability, and increased drug solubility, improved permeability across the BBB, targeted delivery to mitigate systemic side effects, and versatility of incorporation in various delivery methods [44, 49]. Conventional NP drug carriers passively target tumors by the enhanced permeability and retention (EPR) effect in tumor milieu [50,51,52]. These carriers include solid lipid NPs (SLNs), nanostructured lipid carriers, liposomes, dendrimers [53], polymers, micelles, and magnetic and inorganic NPs [54, 55]. Vascular permeability is enhanced in the tumor due to elevated levels of VEGF, peroxynitrite, nitric oxide, and bradykinin, resulting in widened inter-endothelial cell gaps and facilitating NP entry into the tumor site [56]. Moreover, the reduced lymphatic drainage in the tumor increases the retention of NPs and their accumulation [49, 57]. Also, NP surface functionalization aids in their tumor targetability and BBB penetrability. However, conventional NPs are vulnerable to clearance by the reticuloendothelial system (RES) and have off-target effects making the effectiveness of NP drug delivery systems challenging [44, 49, 58].
To address these limitations, recent breakthroughs in bio-imitating natural components of the body have become an area of interest for drug delivery [59]. Biomimetic drug delivery systems (BDDS) mimic biological structures and functions, providing benefits including immune evasion, minimal immunogenicity, lowered toxicity and greater safety than non-biomimetic synthetic NPs, greater accumulation and longer duration of circulation, and biocompatibility targeting drugs through cells, membranes, proteins, and other biological macromolecules [39, 60, 61].
This review provides an overview of biomimetic nanodrug delivery systems (BNDDS) and their application in targeting GBM (Fig. 1). It covers the different types of BNDDS, their mechanisms, and the advantages they offer. The challenges associated with BNDDS are also addressed. Also, an overview on vaccines used in GBM is discussed.
Challenges in drug delivery to brain
Overcoming biological barriers in GBM therapy
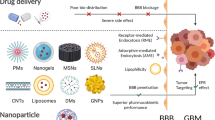
After intravenous injection, nanodrugs travel through a series of cascade processes to take action, including crossing the BBB, building up at the tumor site, infiltrating tissue, undergoing endocytosis, and releasing the drug payload [62]. The anti-tumor activity of these carriers is strongly influenced by the effectiveness of these steps. BNDDS have immense potential in overcoming biological barriers [63] and attaining targeted medication delivery [64, 65]. This section outlines the main biological barriers that nanodrug delivery systems face when delivering drugs to tumor locations and how BNDDS can help overcome such barriers (Fig. 2).
Evading blood barriers
After intravenous injection, NPs face challenges traversing the bloodstream to reach the GBM tumors. They must evade protein adsorption, premature enzyme degradation, and RES clearance [66, 67]. Clearance is influenced by the size, shape, and charged surface of NPs; biomimetic coatings are essential for improving biocompatibility and avoiding clearance [68].
Penetrating the blood–brain barrier and blood–brain tumor barrier
BBB and blood–brain tumor barrier (BBTB) are formidable barriers that limit the ability of chemotherapeutic agents to enter the brain and malignant tissue, respectively [69, 70]. Along the brain and spinal cord's blood vessels, the BBB acts as a highly selective interface, regulating the passage of molecules between the bloodstream and the central nervous system (CNS) to maintain homeostasis [71]. Composed of cerebral endothelial cells (CECs) with tight junctions formed by claudins and occludins [72], the BBB boasts a remarkably higher transendothelial electrical resistance (TEER) than other body tissues. Astrocytes and pericytes play crucial roles in supporting CECs, forming a neurovascular unit that contributes to the integrity of the BBB. The microenvironment surrounding the BBB includes various cell types and the basal membrane, ensuring its rigidity and functionality. Beyond its defensive roles, the BBB regulates the passage of medications into the CNS, often thwarting therapeutic efforts due to multidrug resistance proteins and complex transport mechanisms [73]. Pathological conditions of GBM can disrupt BBB physiology and morphology, leading to variable or partial impairment. This disruption, typically observed at primary tumor sites, contributes to vasogenic brain edema, complicating drug delivery and treatment efficacy. Therapeutic resistance in GBM stems from factors including disturbed BBB, active drug-resistant mechanisms, low blood flow, and high intra-tumoral pressure [74]. The BBTB emerges at the interface between capillary vessels and cerebral tumor tissues, resulting from disruptions in the BBB caused by the progression of GBM [75]. It forms due to tumor membrane breakdown and deterioration, replacing the BBB via angiogenesis and impeding the delivery of drugs. VEGF expression drives angiogenesis in hypoxic tumor areas, enhancing nutrient and oxygen supply but hindering drug penetration. GBM therapy efficacy hinges on the permeability of the neo-vasculature of the tumor, which varies in vessel density and diameter across three phases. The BBTB can become leaky in high-grade gliomas due to their heightened metabolic demands. Still, the barrier remains largely intact, limiting the effectiveness of EPR strategies. The unique microenvironment of gliomas exacerbates this challenge, demonstrating the role of the BBTB in impeding therapeutic agent delivery at effective concentrations [76, 77].
Overcoming the BBB and BBTB are crucial in treating GBM effectively. BNDDS present promising solutions to traverse these barriers [78], employing innovative strategies such as utilizing ligand installed nano-carriers [79], neutrophils [80, 81], or genetically modified cell membranes [82,83,84] with endothelial crossing capabilities to traverse endothelial barriers. NP diffusion is further hampered by the high interstitial pressure in the tumor and malfunctioning blood vessels. Anti-angiogenic drugs can partially alleviate these barriers by normalizing tumor blood vessels and reducing the interstitial pressure [85]. The engineered biomimetic nanocarriers expressing extracellular matrix (ECM)–degrading enzymes enhance NP penetration and cell infiltration into the tumor tissue, facilitating effective delivery of therapeutics to GBM sites [86, 87].
Cellular membrane traversal and intracellular delivery
For therapeutic efficacy, chemotherapeutic drugs encapsulated within NPs must enter tumor cells. However, steric hindrance, dense cell matrices, and electrostatic interactions pose challenges to NP diffusion [88]. Biomimetic NPs offer innovative solutions to enhance cellular membrane traversal and intracellular delivery, utilizing modifications such as peptides that infiltrate tumors [89, 90] and cell-specific recognition ligands [91, 92].
Overcoming multidrug resistance
Multidrug resistance (MDR) mediated by drug efflux pumps remains a major obstacle in GBM therapy [93]. Overexpression of efflux transporters leads to drug expulsion from tumor cells, reducing intracellular drug concentrations and treatment effectiveness [94]. Biomimetic NPs, aided by non-invasive focused ultrasound, efficiently remodel the immunosuppressive microenvironment of glioblastoma, acting as potent checkpoint inhibitors to reduce PD-L1 expression, while NIR II light irradiation converts the tumor from "cold" to "hot," inducing immunological memory to prevent recurrence [95].
Biomimetic nanodrug delivery system
NPs with sizes ranging from 10 to 100 nm in at least one dimension serve as effective medication carriers and aligns well with the dimensions of DNA and proteins [96]. They have two major mechanisms of cellular targeting—active and passive [97, 98]. Passive targeting capitalizes on the characteristics of the TME and the EPR effect [99, 100]. Unlike in normal tissues where NPs are cleared by the mononuclear phagocytic system or kidney filtration, tumors exhibit neovascularization, leading to leaky blood vessels with large pores [101]. This allows macromolecules, including NPs, to accumulate within the tumor tissue. In addition, disrupted lymphatic function in tumors results in minimal fluid uptake, contributing to particle retention in the tumor interstitium via the EPR effect [102]. Nanocarriers exploit properties of the TME such as acidic pH, higher redox potential, and lytic enzyme secretion for uniform drug delivery. The acidic milieu created by glycolytic metabolism of cancer cells triggers pH-sensitive NPs to release drugs near cancer cells [103]. However, passive targeting limitations encompass non-specific drug distribution, inconsistent EPR effect presence, and variable blood vessel permeability among tumor types. In contrast, active targeting employs ligands on NP surfaces to specifically target cancer cells with overexpressed receptors, facilitating receptor-mediated endocytosis for effective drug release. Targeting moieties (monoclonal antibodies, peptides, amino acids, vitamins, and carbohydrates) bind to receptors such as transferrin, folate, glycoproteins, and epidermal growth factor [104].
However, a significant issue concerning the EPR effect of NPs within bodily fluids is the risk of medication leakage into malignant cells. This results from opsonization, where plasma proteins such as complement proteins and immunoglobulin G (IgG) adhere to NP surfaces, prompting the RES to identify and eliminate therapeutic NPs as foreign entities [92]. To overcome this, nano-carriers have undergone significant evolution, developing complex chemical architectures to incorporate specific functionalities that enable them to selectively target desired sites with their payload while evading unwanted immune clearance [105, 106]. Various hydrophilic polymers, including polyethylene glycol (PEG), are commonly used to coat NPs for enhanced evasion of the immune system and amplification of the EPR effect. In addition, proteins, vitamins, peptides, antibodies, and aptamers are utilized as functional ligands to decorate the PEGylated NP surface, aiming to overcome steric hindrance and improve NP compatibility within biological environments [107]. These considerably prolong the duration of NP uptake in vivo, changing it from minutes for NPs without PEG coating to hours for NPs coated with PEG [105]. These ligands are selected for their strong affinity to receptors, especially those overexpressed on tumor cells, enabling precise targeting based on the unique features of the target cells [108]. However, PEGylation presents challenges, as recent research indicates that PEG-coated NPs can be swiftly eliminated by the liver upon repeated dosing, a phenomenon termed "accelerated blood clearance (ABC)" [109]. This is linked to IgM antibodies targeting PEG and PEG-induced complement activation, potentially leading to hypersensitivity reactions in certain instances [84]. However, problems related to compatibility and immunological responses persist, and therefore, researchers have turned to studying human cells and proteins to develop advanced treatment strategies such as cell membrane camouflaging [110]. The use of natural cell membranes (CMs) in a biomimetic camouflage method to enhance tumor-targeted therapy has garnered significant attention in recent years. An innovative BNDDS has surfaced, wherein NPs are encapsulated with CMs produced from biological sources. By maintaining the physicochemical characteristics and drug-carrying ability of the particles, this novel approach aims to maintain the physiological function of the source cells while actively passing through biological barriers like angiogenic tumor vessels, inflamed vessels, and the BBB. This provides the opportunity to get past these barriers without depending on the EPR effect [111]. By mimicking the natural cell membranes, the biomimetic camouflage enables the NPs to evade immune detection and clearance mechanisms, thus prolonging circulation time and enhancing tumor accumulation via active targeting mechanisms. Also, the biomimetic coating provides additional functionalities, such as specific targeting ligands or therapeutic molecules, which can further increase the tumor specificity and efficacy of medication delivery sites [70, 92, 108].
Preparation of BNDDS
Typically, BNDDS consists of drug-loaded core particles encased in biologically active biomimetic outer membranes [70]. The core particles are typically composed of materials classified as inorganic, organic, or hybrids of organic and inorganic matrices. Organic materials such as proteins, polymers, and lipid-based NPs have been approved by the regulatory agencies such as the USFDA and EMA since the 1990s. Because they have distinct electrical, magnetic, and optical properties, inorganic materials like metals, carbon-based NPs, mesoporous silica NPs (MSNs), and metal–organic frameworks provide excellent drug loading rates and biocompatibility. As remarkable organic–inorganic hybrid nanomaterials, metal–organic frameworks combine the benefits of both components. Personalized designs are necessary for a variety of clinical applications due to the significant impact core nanomaterials have on drug release patterns, pharmacokinetics, and the size and form of delivery systems. Biomimetic materials, which serve vital functions in packing, safeguarding, targeting, and improving NP biocompatibility, include natural CMs, CM mimics, functionalized CMs, and CM derivatives (extracellular vesicles). These materials successfully replicate biological systems [105, 108]. Typically, the process involves three main steps for self-assembly [84, 112, 113]. Figure 3 provides an overview of the steps involved in the preparation of BNDDS.
It is crucial to characterize BNDDS post-preparation, with an emphasis on confirming full wrapping of NPs and biomimetic membrane, investigating properties such as morphology, particle size, and surface electrical charge changes of modified NPs. Safety and functionality tests include evaluation of the toxicity, release, and efficacy of functional proteins and encapsulated drugs [114, 115]. However, because of the dynamic and complicated nature of BNDDS upon entry into the body and the variability of the TME, characterization is still difficult [70].
Types of BNDDS
Various types of biomimetic NPs have been investigated based on biomaterials such as red blood cells (RBCs), white blood cells (WBCs), natural killer (NK) cells, macrophages, platelets, extracellular vesicles (EVs), and even cancer cells, which can be employed for coating drug NPs (Fig. 4) [116,117,118,119]. This amplifies their biomimetic capabilities and demonstrates higher efficacy across a spectrum of treatments showcasing prolonged in vivo circulation, precise targeting capabilities, reduced immune system clearance, and promising advancements to clinical trials [92, 108]. In the realm of fundamental biomaterials, biomimetic nanocarriers can be classified into two primary categories: (1) synthetic nanocarriers, which are engineered to replicate biological materials, for example, natural proteins, viral capsids, and monoclonal antibodies, augmented by synthetic counterparts such as aptamers and targeting peptides [92]; (2) biological building blocks like bacteria and viral vectors that have undergone passivation [108]. Cell membranes without cytoplasm and organelles, termed "cell ghosts," exhibit precise markers for NP distribution, mirroring their source cells structurally and functionally. They facilitate direct NP coating without additional chemical modifications, yielding biologically intact bilayer membranes that replicate source cell surfaces. They can actively cross biological barriers such the BBB, angiogenic tumor vessels, and irritated vasculature. Biomimetic DDS can go beyond these barriers without depending on the EPR effect. This enhances nanocarrier biocompatibility, enabling efficient and prolonged in vivo circulation and targeted performance. Thus, incorporating CMs into biomimetic methods offers precise biological identity through structured arrays of membrane proteins, ensuring specific molecular interactions [120]. The stability, content, orientation, and glycosylation of membrane proteins may be impacted by the experimental techniques used in the synthesis of complex NPs, which could have an impact on biological interactions, necessitating investigation of the interaction between biological components and biomimetic NPs [105].
RBC membrane–coated NPs
Advancements in molecular and cellular biology, coupled with nanotechnological progress, have spurred researchers to devise nanocarriers inspired by RBCs, the predominant cellular component of human blood. RBCs are enucleate and measure approximately 7 µm in diameter [121]. Their capacity to alter shape during circulation and their convenient isolation from blood render them an optimal source of cell membranes for in vivo navigation within patients' blood vessels(108, 121). They possess CD47 (self-antigen) protein on their surface, which extends their lifespan in vivo (approximately 120 days in humans and 50 days in mice) [122]. Moreover, their naturally biodegradable, non-toxic, and semipermeable membrane facilitates constant drug release enhances their utilization in coating specific NPs for targeted drug delivery [105]. RBC membrane-covered SLNs, equipped with T7 and NGR peptides and carrying vincristine, demonstrate potent anti-glioma effects by improving drug transport to the brain, overcoming barriers like the BBB and BBTB [105]. In another study, docetaxel (DTX) nanocrystals were encapsulated, and a lipid insertion technique was employed to fabricate T7/NGR-co-modified RBC membrane-coated SLNs, showcasing remarkable tumor cell specificity and robust therapeutic efficacy in orthotopic GBM mouse models [123]. Chai et al. demonstrated through comprehensive in vitro and in vivo studies that CDX peptide (derived from candotoxin)-RBC NPs effectively penetrate the BBB, exhibiting remarkable brain targeting ability. Loading these nanoparticles with doxorubicin (DOX) notably extended the median survival of mice with glioma [124]. Functionalizing RBCM with angiopep-2 and loading pH-sensitive NPs (comprising dextran, DOX, and lexiscan) onto these modified membranes enhanced blood circulation duration and facilitated exceptional penetration through the BBB. Furthermore, in orthotopic U87MG human GBM tumor-bearing nude mice, this approach resulted in heightened accumulation and prolonged retention in the tumor, showcasing promising potential for GBM therapy [125]. Fu et al. engineered a novel RBC-coated SLN (RBCSLN), dual-modified with T7 and NGR peptides and encapsulated with vincristine. These demonstrated superior anti-glioma effects in vitro and in vivo, resulting in dual-targeted delivery [126]. Furthermore, a nanogel formulation incorporating miR155 NPs coated with RBCM was found to extend the circulation lifetime of the microRNA, while also imparting active tumor-targeting ability and efficacy in inhibiting GBM [127, 128].
WBC membrane–coated NPs
Leukocytes possessing unique characteristics have emerged as significant carriers for targeted drug delivery. They induce inflammation in tumor areas, promote vascular permeability, facilitate particle movement, and evade immune surveillance, making them crucial for targeted administration [110]. Larger than RBCs, they exhibit rapid and efficient movement from the bloodstream into surrounding tissues, making them abundant, both within blood vessels and outside them. Their adhesive properties enable direct interaction with tumor cells either within the cancerous environment or in the bloodstream [70, 105]. Of the five primary classes of leukocytes (lymphocytes, monocytes/macrophages, neutrophils, eosinophils, and basophils), macrophages [129] and neutrophils are highly utilized [84, 130]. Macrophages, with surface markers like CD11b or CD49d, possess the ability to interact with chemokines and inflammatory factors within tumor tissues, enabling specific targeting of tumor cells [130]. This capability allows for precise drug delivery to target sites such as tumors and inflamed tissues. Moreover, the controlled release profiles offered by NPs facilitate tailored treatment strategies for optimized therapeutic outcomes [110]. In addition, tumor-associated macrophages, influenced by their polarization state, tumor type, and disease stage, can serve both as therapeutic targets and as carriers for drug delivery, demonstrating their versatile roles in cancer treatment [70]. NPs coated with macrophage membranes possess the unique ability to seamlessly traverse between blood vessels and extravascular tissues, facilitated by their biocompatibility and immune evasion mechanism conferred by CD47 embedded within the membranes [131]. An engineered system consisting of macrophage membrane-coated poly (lactic-co-glycolic acid) (PLGA) NPs containing DOX showed better transport through the BBB and enhanced therapeutic efficacy in GBM due to enhanced expression of programmed cell death-1 (PD-1) [132]. A study demonstrated the effectiveness of DSPE-PEG NPs loaded with near-infrared Ib (NIR-Ib) fluorescent dye IR-792, decorated with macrophage membrane, in crossing the BBB and selectively accumulating at the tumor site. This approach enables a combination of NIR-Ib imaging and NIR-Ib imaging-guided photothermal therapy, leading to significant inhibition of glioma growth [133]. In vitro studies confirmed that silica NPs containing DOX, coated with macrophage membrane, exhibit enhanced cellular uptake and penetration into the core of glioma spheroids compared to bare NPs. In addition, in nude mice with intracranial U87 glioma, loading NPs into macrophages significantly improved their tumor-targeting efficiency [131]. Another study utilized DOX-loaded NPs covered with monocytes which improved tumor drug delivery efficacy and damage-associated molecular patterns emission via BBB penetration and GBM infiltration [127]. Neutrophils, constituting over 50% of leukocytes, serve as initial responders in acute inflammation, making them promising candidates for targeted delivery systems [130]. Activated neutrophils migrate toward inflammatory sites guided by chemotactic gradients, engaging in pathogen elimination via phagocytosis. Furthermore, neutrophils possess the ability to traverse the BBB or BBTB, infiltrating tumor masses [134]. Xue et al. showcased that neutrophils carrying paclitaxel (PTX) liposomes retained their physiological activity and migrated to the inflamed brain tumor, consequently enhancing the survival of mice [81]. Also, zoledronate NPs wrapped in macrophage membrane performed better than bare ones in GBM orthotopic mice model [135].
Platelet cell membrane–coated NPs
Platelet membranes, derived from megakaryocyte progenitors, offer remarkable physiological functions and are readily available in large quantities, making them of significant interest as platforms for cancer targeting. Platelets, which can be used to coat NPs, possess functions beyond hemostasis. They target injured tissues and tumor sites via various surface marker such as CD47, CD55/59, CD44, and P-selectin receptors, enabling immune evasion, preventing complement activation, and binding to circulating cancer cells [70, 105]. Despite their superior targeting ability compared to RBCs, platelets face challenges due to their limited proportion in blood and poor stability, hampering their clinical translation [105]. A study proposed that MEDI-575, an immunoglobulin G2K monoclonal antibody, exhibits high specificity in binding to platelet-derived growth factor-α receptor (PDGFR-α), thereby reducing the growth of GBM tumors [136]. Another strategy employing DOX-loaded pH/redox dual-responsive nanogels (DOX@PNGs) targeted orthotopic GBM, enhancing the therapeutic efficacy of the drug. This functional drug delivery system was targeted to the tumor site through interactions with membrane surface proteins, releasing DOX promptly in response to the TME. In vivo tests demonstrated the remarkable targeting effects of the system and increased survival time [137]. Loading quercetin inside platelets resulted in enhanced anti-tumor activity, with greater inhibition of U373-MG tumor cells observed (14.52 ± 1.53% cell viability) compared to free quercetin (21.99 ± 2.09% cell viability) [138].
Cancer cell membrane–coated NPs
Cancer cell membranes (CCMs) are easily cultured in vitro, providing abundant membranes with inherent homologous targeting (unlike most other membranes) and antigenic library functions, allowing for natural targeting without complex modifications [139]. Adhesion molecules on CCMs, such as integrins, selectins, and E-cadherins, facilitate cancer development and metastasis by mediating cell–cell adhesive contacts. CD47, overexpressed on tumor cells, promotes immune evasion by blocking phagocytosis [140]. CCM-coated NPs (CCM-NPs) exploit these mechanisms, offering immune evasion through CD47, homotypic targeting via cadherin and integrins, and cancer immunotherapy through vaccination, leading to enhanced tumor-specific accumulation, longer circulation, and efficient drug or gene delivery while stimulating immune responses against tumor-associated antigens to elicit antitumor effects [141, 142]. Also, through increased photothermal therapy efficacy and magnetic particle imaging sensitivity in animal models, the CCM-coated SPIO nanoprobe improved early-stage glioma detection and treatment [143]. CCM-NPs exhibited a 40-fold and 20-fold increase in uptake in MDA-MB-435 cells compared to RBC-coated NPs and bare PLGA cores, respectively, underscoring their affinity for cancer cells due to cancer cell adhesion molecules from cancer CMs [139]. Pasquale et al. developed DOX-loaded boron nitride nanotubes (BNNTs) coated with GBM cell membranes (DOX-CM-BNNTs), demonstrating their specific targeting and killing of GBM cells in vitro, while sparing healthy brain cells after crossing an in vitro BBB model [144]. A hybrid nanocube, comprising an inorganic core (Fe3O4/MnO2) enveloped with cell membranes derived from the U-251 MG cell line, displayed favorable magnetic properties and NMR relaxation times. This suggests their promising utility as theranostic agents for GBM [145, 146]. Magnetic NPs (MNPs) efficiently co-load TMZ and cisplatin (CDDP), crossing the BBB to target GBM specifically. Mice with orthotopic U87MG or drug-resistant U251R GBM tumors treated with MNPs@TMZ + CDDP exhibit potent anti-GBM effects, significantly extending survival compared to single-drug loaded NPs [147]. Sorafenib encapsulated within iron oxide and manganese oxide NPs, coated with GBM cell membrane, facilitated homologous targeting, resulting in increased apoptosis and necrosis in GBM cells [148].
Stem cell membrane–coated NPs
Stem cells, including mesenchymal stem cells (MSCs), harvested from diverse tissues like adipose tissue, peripheral blood, placenta and umbilical cord, possess exceptional self-renewal abilities and support various cell types. MSCs exhibit unique biological properties and in vitro proliferation, making them suitable for in vivo applications. With advantages such as prolonged circulation, immune evasion, and inherent cancer-targeting capabilities, MSCs are well suited for delivering biomimetic NPs [149]. These capabilities stem from specific ligands expressed by MSCs, facilitating precise targeting of cancerous and damaged tissues in vivo. Coating NPs with membranes derived from MSCs offers a promising approach for targeted tumor treatment, leveraging their abundance of targeting molecules and innate homing capacity. MSCs use complex signaling networks to interact with not only the tumor cells, but also with the TME and with the immune system, thus demonstrating inflammatory tendency and tumor targetability. Enveloping NPs with MSC membranes heightens their biocompatibility and also their therapeutic effect of NPs by simulating MSCs' targeting capabilities. Different sources of MSCs have varying therapeutic potentials due to variations in content, accessibility, proliferation, cytokine profiles, and immunoregulation [70, 105]. Chang et al. genetically engineered 150 human pluripotent SCs using CRISPR/Cas9 to express the anti-GBM–chimeric antigen receptor (CAR) constructs with T-specific CD3 + or neo-genin specific γ-signaling domains. The CAR vectors demonstrated effective anti-tumor activity against GBM, delivering TME-responsive NPs specifically and non-invasively. This combination therapy significantly extended the lifespan of female mice with tumors. In another study, MSCs/NP systems showed a faster migration rate toward malignant glioma cells (U87) compared to single nanocomposites [140]. Yen et al. and Lai et al. developed stem cell-NP systems (SNS) for GBM targeting and improvement in gadolinium-neutron capture therapy (Gd-NCT), using magnetized UMSCs loaded with gadodiamide-concealed MNPs (GdFPFNP), leading to improved treatment efficacy and extended survival in orthotopic GBM rat models [150, 151].
Hybrid membrane-camouflaged NPs
To enhance NP properties and address limitations in single-cell membrane disguises, researchers fuse multiple cell membranes to optimize tumor targeting and tissue penetration, and minimize toxic effects, achieving better drug and NP distribution and therapeutic outcomes [141]. Platelet–cancer cell hybrid membrane-coated hollow PLGA NPs loaded with β-mangostin exhibit enhanced anticancer efficacy against glioma cells, offering homotypic cell targeting, immune escape, sustained tumor growth inhibition, metastasis suppression, and excellent biocompatibility, making them promising glioma treatment candidates [152]. A biomimetic nanosystem (HM-NPs@G) with coating of cancer cell–mitochondria hybrid membranes (HM) on gboxin-loaded NPs demonstrates enhanced biocompatibility, pharmacokinetic profile, BBB permeability, and homotypic dual targeting, leading to improved blood circulation (4.90 h vs 0.47 h for free gboxin) and tumor accumulation (7.73% ID/g vs 1.06% ID/g for free gboxin) [153].
Cell-mediated NPs
Endogenous cells possess innate capabilities to breach the blood–brain barrier and infiltrate tumor sites, rendering them potential carriers for brain-targeted drug delivery systems. Immunocytes, including mononuclear phagocytes, lymphocytes, and neutrophils, alongside stem cells, exhibit homing properties enabling migration to injury, inflammation, and tumor locations [154, 155]. Xue et al. utilized neutrophils as carriers for PTX-loaded liposomes to enhance brain-targeted delivery and effectively suppress postoperative glioma recurrence [81]. Utilizing dendritic cells and macrophages as delivery vehicles presents a promising strategy for circumventing the BBB and overcoming various structural and metabolic hurdles that typically hinder drug penetration into GBM [156]. Yu et al. created a dendritic cell–mediated nano-DOX system to boost anti-GBM immune response [157], while Hao et al. developed stem cell–delivered nanogels for enhanced tumor MRI [158].
Extracellular nanovesicle-camouflaged NPs
Extracellular vesicles (EVs), like exosomes and microbubbles, play vital roles in cell communication and disease processes. While they hold promise as lipid bilayer nanocarriers for cancer treatment due to their compatibility with organisms, low toxicity, and high drug capacity, their industrial production remains limited [159]. Despite challenges, engineered exosomes show potential for enhanced drug delivery through various modifications. However, addressing complexities in preparation, understanding mechanisms, and improving production efficiency are essential for realizing their clinical potential [160]. Research has validated the anti-GBM efficacy of ESC-exosomes, showcasing their effectiveness compared to alternative drug carriers [161]. Patient-derived GBM cell lines were used in the investigation by Araujo et al. to identify and thoroughly characterize EVs. The overall amount of medications required to cause an effect on tumor cells was shown to be reduced after loading them with two distinct pharmaceuticals, TMZ and EPZ015666 [162]. Niu et al. pioneered a biomimetic drug delivery system by combining natural grapefruit EVs with DOX-loaded heparin-based NPs, demonstrating high efficiency in glioma treatment [163]. Zhu et al. utilized endogenous embryonic stem cell–derived exosomes as drug carriers, confirming their efficacy against GBM and preparing c(RGDyK)-modified, PTX-loaded exosomes (cRGD-Exo-PTX) [164]. A tailored delivery system, employing angiopep-2 and TAT peptide-functionalized small EVs, effectively breaches the BBB, targets gliomas, infiltrates tumors, and enables precise chemotherapy, reducing drug-induced side effects [70].
Virus-based NPs
Viral vectors have been pivotal in glioma gene therapy trials over the past 25 years, with retroviral (e.g., Toca 511) and adenoviral vectors (e.g., Ad-p53) showing promise despite limited clinical translation due to challenges in tumor penetration and survival increases. To address these issues, engineered viral vectors like adeno-associated virus (AAV) and plant viruses, such as cowpea mosaic virus (CPMV), are emerging as promising options for brain tumor treatment [165]. Lam et al. developed novel CPMV-based NPs for targeted delivery of mitoxantrone, demonstrating effective uptake and retained therapeutic efficacy in GBM cells, addressing challenges associated with systemic delivery [166]. A hepatitis B core protein-virus-like particle (VLP)-based dual-targeting delivery system was engineered, employing brain-targeting peptide TGN for BBB penetration and GBM-targeting ligand RGD. This system efficiently co-delivered PTX and siRNA to invasive tumor sites, resulting in synergistic antitumor effects, including enhanced necrosis and apoptosis, and reduced tumor invasion with minimal cytotoxicity, showing promise for GBM therapy [167]. Virotherapy including oncolytic viruses [168] employs Newcastle Disease Virus (NDV) alongside TMZ-PLGA-NPs, which demonstrated synergistic antitumor effects against GBM. The combination therapy, evaluated in vitro on AMGM5 GBM cells, revealed higher cytotoxicity and inhibition of colony formation than individual treatments [169]. The study utilized three virus-like particles (VLPs): MS2 spheres, tobacco mosaic virus (TMV) disks, and nanophage filamentous rods, modified with DOX. While all VLPs demonstrated effective drug delivery and cell uptake in vitro, glioma-bearing mice treated via convection-enhanced delivery with TMV disks and MS2 spheres conjugated to DOX showed increased survival rates. This was demonstrated particularly in TMV-treated mice, following a single VLP-DOX CED injection at significantly lower doses compared to traditional intravenous doses [170]. C6 GBM CM-coated NPs efficiently delivered PEI25k/pDNA (polyethylenimine) complexes, demonstrating high transfection efficiency, low toxicity, and enhanced therapeutic effects in GBM models [171]. Sendai virus enabled efficient cytosolic delivery of quantum dots in GBM cell cultures, reducing nonspecific endocytosis by 50% as demonstrated by fluorescence microscopy and transmission electron microscopy [172].
Protein-based NPs
Proteins and peptides play crucial roles in brain function, regulating cerebral blood flow, BBB permeability, neurotransmission, neuromodulation, and immune responses, making them promising candidates for brain-targeted drug delivery systems [173]. Their inherent biodegradability, biocompatibility, low toxicity, and ease of modification make protein-based drug delivery systems advantageous [141, 174]. Consequently, protein nanocarriers have garnered significant attention as potential drug delivery systems for brain tumors. The cRGD peptide selectively binds to GBM cells, and the results showed that the uptake of cRGD by bovine serum albumin and human serum albumin (BSA/HSADOX) in tumor cells was significantly enhanced [175]. Gregory et al. developed synthetic protein NPs (SPNPs) using polymerized human serum albumin (HSA) and the cell-penetrating peptide iRGD, capable of delivering siRNA against STAT3 (oncogenic signal transducer and activator of transcription 3) in GBM. This approach leads to STAT3 downregulation in vitro and in vivo, resulting in tumor regression and prolonged survival when combined with radiation therapy, while also inducing anti-GBM immunological memory in mice [176]. Temozolomide acid (TMZA)–loaded HSA NPs were developed targeting GBM and brain cancer stem cells (CSCs), demonstrating efficient cellular uptake and high cytotoxicity in vitro and in vivo [177]. Evaluation of LinTT1, a tumor-penetrating peptide targeting cell surface p32 protein, demonstrated enhanced tumor homing of systemically administered NPs in various GBM models, suggesting its potential for improving imaging and therapy in GBM [178]. IL-13 receptor alpha 2 (IL-13Rα2)–targeted PEGylated-polycaprolactone (PCL) NPs loaded with docetaxel exhibit increased anticancer activity in glioma-bearing murine models by enhancing cellular uptake and glioma localization. Also, angiopep-2-conjugated PEG-PCL NPs exhibited enhanced glioma targeting and BBB penetration, leading to improved survival and safety profile in glioma-challenged mice compared to conventional therapies [179].
Challenges with biomimetic NPs
Notwithstanding advancements in BNDDS, there are constraints and safety concerns such as biocompatibility, targeting specificity, stability, biological interactions, and ethical considerations [180]. Most research is being done in laboratory settings, but because species variations between humans and animal models have a big influence on biomimetic NPs, it is crucial to take such differences into account. Limited research exists on the impact of tumor behavior and function, necessitating the development of models reflecting tumor heterogeneity for tailored treatment strategies [181]. The clinical utility of biomimetic NPs faces challenges [182] such as limited scalability due to early-stage research [183], restricted characterization methods [184], and safety concerns, necessitating meticulous refinement for successful clinical translation [185]. Recent research has predominantly focused on the accumulation of biomimetic NPs at tumor sites, overlooking the impact of its physical and chemical properties on interactions with living organisms, with limited attention to lifespan and quality of life. In addition, conformational changes in surface-trapped proteins were correlated with increased toxicity [92]. Even though biomimetic NPs made from different cell sources have the potential to overcome problems with administering free therapeutic molecules such as low solubility, non-specific targeting of cancer cells, and consequent negative impacts on healthy cells, it is still important to address its toxicity and biological impact. These nano-bio hybrid NPs seek to evade RES filtering and maintain extended circulation, which may have unanticipated negative effects [186]. Standardizing NP manufacturing experimental protocols between laboratories is crucial to guarantee reproducibility and expedite their clinical translation. Variations in experimental techniques, however, may affect the characteristics of the membrane, which could lead to immunological reactions and unfavorable outcomes because of modifications in the stability, composition, orientation, and glycosylation of the proteins. Furthermore, the range of applications for membrane-coated NPs is growing, thanks to novel techniques like lipid insertion, transmembrane hybridization, biological engineering, and genetic manipulation [187]. Standardized procedures, rigorous biocompatibility assessment, and ongoing advancements in NP design are essential to address these challenges, potentially revolutionizing targeted drug delivery and improving patient outcomes in oncology and beyond.
Vaccines for GBM
With advancements in vaccination platforms and mechanistic investigations, cancer vaccination, a promising immunotherapeutic strategy against solid tumors, has undergone tremendous evolution. Cancer vaccines work to overcome tumor immunosuppression and improve antitumor immunity by providing tumor specific antigens (TSA) through a variety of modalities, including entire cells, peptides, and nucleic acids [180]. Preclinical research has yielded encouraging results, but effective phase III studies for GBM are still elusive [181, 182].
Peptide vaccines
TSAs produced by the many mutations in GBM trigger immunological responses. Nevertheless, because of their high epitope expression in GBM and low specificity, non-mutational antigens increase the danger of autoimmunity, impeding peptide vaccine–based approaches [183]. Targeted by the CDX-110 peptide vaccine, the most common TSA in GBM is the mutated EGFRvIII, which is present in 20–30% of cases. Despite eliciting a robust immune response, the phase III trial (ACT IV) failed to significantly enhance overall survival, despite showing promise in preclinical models. The progress of EGFRvIII-targeting treatments such as ADU-623 was hampered by issues such as low threshold values for EGFRvIII detection and unsatisfactory outcomes due to the removal of EGFRvIII-expressing tumor cells as well as the natural decline of these cells [184, 185]. TSAs that show promise include mutated isoenzyme dehydrogenase (IDH), which are not present in normal cells. One such mutation is the R132H mutation in IDH1, which is commonly found in secondary low-grade gliomas. Phase I clinical trials are currently being conducted on peptide vaccines that target IDH1 R132H, which induces antigen-specific CD4 + T cells and humoral responses [186]. Novel antigens chosen by whole exon sequence comparison are the subject of recent experiments aimed at developing tailored cancer vaccines. The promise for customized vaccination approaches is highlighted by these trials, which produce sizable numbers of tumor-reactive T memory cells [187]. Heat shock proteins (HSPs) are overexpressed in certain malignancies, such as GBM, and they have roles in cellular defense. Combining HSPs with tumor antigens, HSP vaccines like HSPPC-96 have shown potential in clinical trials for the treatment of GBM. HSPPC-96 improved median progression-free survival (PFS) and overall survival (OS) compared to standard therapy in phase I/II trials, demonstrating safety and efficacy. Trials are ongoing to learn more about HSPPC-96's efficacy and mechanism in GBM treatment [188].
Tumor cell–based vaccines
Early GBM vaccines that used inactivated or killed tumor cells have shown low success rates. To improve vaccination efficacy, gene-edited tumor cells producing immune-stimulating cytokines such as granulocyte macrophage colony stimulating factor (GM-CSF) were introduced in the late 1980s. Promising results from phase I studies utilizing autologous and allogeneic tumor cells secreting GM-CSF have highlighted the significance of T-cell activation in anti-tumor immunity. Furthermore, formalin-fixed GBM is being directly injected as an antigen; a clinical trial using DC vaccinations demonstrated an overall survival of 22.2 months [183, 189].
Dendritic cell–based vaccines
Dendritic cell (DC) vaccines are the subject of ongoing GBM vaccination studies, which take advantage of DCs' strong antigen-presenting capabilities. Using patient DCs loaded with tumor antigens, these vaccines are designed to strengthen the immune system. Production costs are substantial and there is inconclusive evidence for phase III efficacy despite encouraging results. Notably, phase III trials for Northwest Biotherapeutics' Diva project show advancements in the development of DC vaccines [183]. When Liau et al. used DC vaccines for the first time in the treatment of GBM in 2000, a patient with recurrent brainstem glioma was able to live an additional 21 months [190]. Yu et al. achieved a median OS of 133 weeks for patients with recurrent GBM by loading DCs with peptides from autologous glioma cells in 2001 and 2004 [188]. When Okada et al. created vaccines using tumor lysates in 2007, the safety profiles and immune responses were better. With grade 1 or 2 toxicities noted, more than ten phase I/II trials have shown the viability of DC vaccines, primarily using tumor lysate-pulsed DCs. A median PFS of 10.4 months and a median OS of 18.3 months were achieved by incorporating the DC vaccine into the Stupp regimen through the HGG-2006 study. However, with 39% of participants having grade 3/4/5 adverse events, HGG-2006 adverse events were more severe than with other DC vaccines studies [191]. In 2013, Prins et al. conducted a comparison between glioma-associated antigen (GAA) peptide-pulsed DC vaccination and autologous tumor lysate (ATL)–pulsed DC vaccination. The results indicated that ATL-pulsed DC vaccination produced greater activation of NK cells [188].
Gene-based vaccines
Genetic vaccines, utilizing nucleic acids like DNA or RNA, prompt cells to produce specific proteins, triggering an immune response against tumors [182] with mRNA-based vaccines gaining traction due to their low infection risk, rapid degradation in the body, and potential for tailored cancer treatments through modifications extending their half-life [192, 193]. Glioma antigens such as ANXA5, FKBP10, MSN, and PYGL are promising and have the potential to be developed into mRNA vaccines [194, 195]. The effectiveness of mRNA vaccines against GBM is being studied in clinical trials such as NCT02465268 [183, 196], with promising outcomes including better OS and PFS as compared to conventional therapy. Other trials, such as NCT00846456 [197], use mRNA derived from autologous GBM stem cells to target CSCs, and they demonstrate a significant improvement in PFS without any major side effects. The development of DNA vaccines, utilizing synthetic DNA plasmids, presents a promising treatment avenue for GBM patients, offering stability, scalability, and human compatibility, with safety advantages over recombinant proteins and viral vectors, capable of eliciting robust immunological responses, including CD4 and CD8 T-cell responses, without inducing immune reactions against the DNA backbone [198]. In a phase I trial for recurrent glioblastoma, VXM01, which encodes vascular endothelial growth factor-2 (VEGFR-2), showed safety and tolerability. It was produced from an attenuated strain of Salmonella typhi. Following immunization, the majority of patients showed increased tumor-infiltrating T cells and T-cell responses specific to VEGFR-2 [199].
Despite promising animal model results, GBM vaccination clinical trials have limited success, attributed to factors such as tumor dedifferentiation, limited CNS drug access, immunosuppression, tumor heterogeneity, and low mutational burden. Immune evasion, resistance to cytotoxic T lymphocyte (CTL)–mediated lysis, and vaccine-associated toxicity further hinder efficacy [181, 183]. Personalized vaccines for GBM, developed over two decades, aim to target individual tumor mutations. However, early trials using tumor lysates showed increased immune response without significant survival improvement. Yet, personalized neoantigen design holds promise for enhancing vaccine efficacy by integrating diverse tumor antigens to reduce tumor load and counteract antigen loss risk. Combining neoantigens and TAAs boosts vaccine effects, extending survival in clinical trials. Long-length peptide vaccine designs minimize T-cell sequestration, reduce exhaustion, and enhance anti-tumor responses, while short-lived vaccines degrade quickly but can be supplemented with immunostimulatory agents [182, 187, 200]. Vaccine adjuvants enhance immune responses, with poly-ICLC (an RNA and polymer complex) and stimulator of interferon genes (STING) agonists showing promise. Prime CD4 + T cells maintain CD8 + T-cell populations and promote effective antitumor immunity, supporting CD8 + T-cell cytotoxicity and eliminating major histocompatibility class (MHC)–deficient tumor cells. Optimizing vaccine design, overcoming immunosuppression, and enhancing patient immunity are crucial challenges, with combination therapies like immune checkpoint blockade offering promise. Future research should compare vaccine platforms and antigen sources to determine effectiveness [201].
Conclusion
In conclusion, tailored medication delivery for a variety of disorders, most notably cancer, appears promising thanks to nanomedicine and the development of BNDDS. Targeted delivery and fewer adverse effects are provided by the biomimetic drug delivery system, which imitates natural particles. Among the discussed types of biomimetic NPs, cell membrane–coated NPs, as those derived from cancer, white blood, or red blood cells, hold great promise for improving the efficacy and specificity of drug delivery systems for the treatment of cancer due to their enhanced biocompatibility, prolonged in vivo circulation, and precise targeting capabilities. However, clinical studies, long-term safety, and drug release mechanisms are among the study areas for BNDDS that require validation, despite the benefits in efficacy and safety. Prolonged circulation and targeted administration are made possible by BNDDS using natural CMs, different endogenous materials, and other cellular components. Although biomimetic membranes can encapsulate a variety of components, including metal NPs and polymers, clinical translation still presents challenges. Many vaccines have been investigated for GBM; to maximize BNDDS's potential, multidisciplinary research, safety evaluations, and clinical trials should be given top priority in the future. To generate reproducible nanocarriers and accelerate their clinical translation, standardizing experimental procedures for creating biomimetic NPs across research sites is crucial. This will help to overcome possible issues related to variability in production processes. To overcome obstacles and fully utilize the potential of this innovative drug delivery technology, more material science and molecular biology research is therefore essential.
Data availability
No datasets were generated or analysed during the current study.
References
Oronsky B, Reid TR, Oronsky A, Sandhu N, Knox SJ (2021) A review of newly diagnosed glioblastoma. Front Oncol 10:574012
Rong L, Li N, Zhang Z (2022) Emerging therapies for glioblastoma: current state and future directions. J Experimental Clin Cancer Res 4
Nørøxe DS, Poulsen HS, Lassen U (2016) Hallmarks of glioblastoma: a systematic review, vol 1. BMJ Publishing Group, ESMO Open
D’Alessio A, Proietti G, Sica G, Scicchitano BM (2019) Pathological and molecular features of glioblastoma and its peritumoral tissue. Cancers (Basel) 11(4):67–89
Tan C, Wei Y, Ding X, Han C, Sun Z, Wang C (2022) Cell senescence-associated genes predict the malignant characteristics of glioblastoma. Cancer Cell Int 22(1):411
Tan AC, Ashley DM, López GY, Malinzak M, Friedman HS, Khasraw M (2020) Management of glioblastoma: state of the art and future directions. CA Cancer J Clin 70(4):299–312
Braganza MZ, Kitahara CM, Berrington de González A, Inskip PD, Johnson KJ, Rajaraman P (2012) Ionizing radiation and the risk of brain and central nervous system tumors: a systematic review. Neuro Oncol 14(11):1316–24
Ostrom QT, Adel Fahmideh M, Cote DJ, Muskens IS, Schraw JM, Scheurer ME et al (2019) Risk factors for childhood and adult primary brain tumors. Neuro Oncol 21(11):1357–1375
Smith C, Perfetti T, Chokshi C, Venugopal C, Ashford J, Singh S (2024) Risk factors for glioblastoma are shared by other brain tumor types. Hum Exp Toxicol 23:43
Gudbergsson JM, Christensen E, Kostrikov S, Moos T, Duroux M, Kjær A et al (2020) Conventional treatment of glioblastoma reveals persistent CD44(+) subpopulations. Mol Neurobiol 57(9):3943–3955
Chen Q, Tan K, Lin Q, Sarah N, Li-Wen Y, Heng S et al (2022) Nanotechnology: a better diagnosis and treatment strategy for brain tumour? J Young Investig 25(3):33–47
Shields LBE, Shelton BJ, Shearer AJ, Chen L, Sun DA, Parsons S et al (2015) Dexamethasone administration during definitive radiation and temozolomide renders a poor prognosis in a retrospective analysis of newly diagnosed glioblastoma patients. Radiat Oncol 10(1):222
Cruz JVR, Batista C, de Afonso BH, Alexandre-Moreira MS, Dubois LG, Pontes B et al (2022) Obstacles to glioblastoma treatment two decades after temozolomide. Cancers (Basel) 14(13):3203
Yang Y, Cheng N, Luo Q, Shao N, Ma X, Chen J et al (2023) How nanotherapeutic platforms play a key role in glioma? A comprehensive review of literature. Int J Nanomedicine 18(July):3663–3694
Rominiyi O, Vanderlinden A, Clenton SJ, Bridgewater C, Al-Tamimi Y, Collis SJ (2021) Tumour treating fields therapy for glioblastoma: current advances and future directions. Br J Cancer 124(4):697–709
Jain KK (2018) A critical overview of targeted therapies for glioblastoma. Front Oncol 8:419
Vaz-Salgado MA, Villamayor M, Albarrán V, Alía V, Sotoca P, Chamorro J, Rosero D, Barrill AM, Martín M, Fernandez E, Gutierrez JA (2023) Recurrent glioblastoma: a review of the treatment options. Cancers 15(17):4279
Birzu C, French P, Caccese M, Cerretti G, Idbaih A, Zagonel V et al (2020) Recurrent glioblastoma: from molecular landscape to new treatment perspectives. Cancers (Basel) 13(1):77–89
Repurposed Drugs in Research for Cancer Clinical Trials - Pitavastatin. ClinicalTrials.gov. Identifier: NCT05977738. Accessed 18 Apr 2024
Surgical nivolumab and ipilimumab for recurrent GBM. ClinicalTrials.gov. Identifier: NCT04606316. Accessed 18 Apr 2024
A proof-of-concept clinical trial assessing the safety of the coordinated undermining of survival paths by 9 repurposed drugs combined with metronomic temozolomide (CUSP9v3 treatment protocol) for recurrent glioblastoma. ClinicalTrials.gov. Identifier: NCT02770378. Accessed 18 Apr 2024
Phase-1 study of folinic acid to modulate MGMT gene in glioblastoma. ClinicalTrials.gov. Identifier: NCT01700569. Accessed 18 Apr 2024
Pilot study of anlotinib with STUPP regimen for patients with newly diagnosed glioblastoma. ClinicalTrials.gov. Identifier: NCT04119674. Accessed 18 Apr 2024
Trial of Enzastaurin and bevacizumab in participants with recurrent malignant gliomas. ClinicalTrials.gov. Identifier: NCT00586508. Accessed 18 Apr 2024
A phase I study to investigate tolerability and efficacy of ALECSAT administered to glioblastoma multiforme patients. ClinicalTrials.gov. Identifier: NCT01588769. Accessed 18 Apr 2024
Efficacy and safety of imatinib mesylate plus hydroxyurea (HU) in patients with recurrent glioblastoma multiforme (GBM). ClinicalTrials.gov. Identifier: NCT00290771. Accessed 18 Apr 2024
Individualized Screening Trial of Innovative Glioblastoma Therapy (INSIGhT). ClinicalTrials.gov. Identifier: NCT02977780. Accessed 18 Apr 2024.
Immunogene-modified T (IgT) cells against glioblastoma multiforme. ClinicalTrials.gov. Identifier: NCT03170141. Accessed 18 Apr 2024
Parvovirus H-1 (ParvOryx) in patients with progressive primary or recurrent glioblastoma multiforme. ClinicalTrials.gov. Identifier: NCT01301430. Accessed on 18 Apr 2024
Dose-escalation study of carboplatin administration into the brain for glioblastoma multiforme. ClinicalTrials.gov. Identifier: NCT01317212. Accessed 18 Apr 2024
Biomarker-driven therapy using immune activators with nivolumab in patients with first recurrence of glioblastoma. ClinicalTrials.gov. Identifier: NCT03707457. Accessed 18 Apr 2024
Super-selective intraarterial intracranial infusion of bevacizumab (avastin) for glioblastoma multiforme. ClinicalTrials.gov. Identifier: NCT02285959. Accessed 18 Apr 2024
Pilot study of autologous chimeric switch receptor modified t cells in recurrent glioblastoma multiforme. ClinicalTrials.gov. Identifier: NCT02937844. Accessed 18 Apr 2024
Nguyen HM, Guz-Montgomery K, Lowe DB, Saha D (2021) Pathogenetic features and current management of glioblastoma. Cancers 13(4):856
Mathur R, Wang Q, Schupp PG, Nikolic A, Hilz S, Hong C et al (2024) Glioblastoma evolution and heterogeneity from a 3D whole-tumor perspective. Cell 187(2):446-463.e16
Becker AP, Sells BE, Haque SJ, Chakravarti A (2021) Tumor heterogeneity in glioblastomas: from light microscopy to molecular pathology. Cancers 13(4):761
Rosenblum D, Joshi N, Tao W, Karp JM, Peer D (2018) Progress and challenges towards targeted delivery of cancer therapeutics. Nature Commun 9(1):1410
Denison TA, Bae YH (2012) Tumor heterogeneity and its implication for drug delivery. J Control Release 164(2):187–191
Wang R, Yan H, Yu A, Ye L, Zhai G (2021) Cancer targeted biomimetic drug delivery system. J Drug Delivery Sci Technol 63:102530
Al-Ostoot FH, Salah S, Khamees HA, Khanum SA (2021) Tumor angiogenesis: current challenges and therapeutic opportunities. Cancer Treat Res Commun 28:100422
Tee D, Distefano J (2004) Simulation of tumor-induced angiogenesis and its response to anti-angiogenic drug treatment: mode of drug delivery and clearance rate dependencies. J Cancer Res Clin Oncol 1(130):15–24
Lugano R, Ramachandran M, Dimberg A (2020) Tumor angiogenesis: causes, consequences, challenges and opportunities. Cell Mol Life Sci 77(9):1745–1770
Singh N, Miner A, Hennis L, Mittal S (2021) Mechanisms of temozolomide resistance in glioblastoma - a comprehensive review. Cancer Drug Resist 4(1):17–43
Lim XY, Capinpin SM, Bolem N, Foo AS, Yip WC, Kumar AP, Teh DB (2023) Biomimetic nanotherapeutics for targeted drug delivery to glioblastoma multiforme. Bioeng Transla Med 8(3):e10483
Chen YX, Wei CX, Lyu YQ, Chen HZ, Jiang G, Gao XL (2020) Biomimetic drug-delivery systems for the management of brain diseases. Biomater Sci 8:1073–88
van Solinge TS, Nieland L, Chiocca EA, Broekman MLD (2022) Advances in local therapy for glioblastoma — taking the fight to the tumour. Nat Rev Neurol 18:221–36
Wilhelm S, Tavares AJ, Dai Q, Ohta S, Audet J, Dvorak HF et al (2016) Analysis of nanoparticle delivery to tumours. Nat Rev Mater 1:16014
Alrushaid N, Khan FA, Al-Suhaimi EA, Elaissari A (2023) Nanotechnology in cancer diagnosis and treatment. Pharmaceutics 15(3):1025
Beh CY, Prajnamitra RP, Chen LL, Hsieh PCH (2021) Advances in biomimetic nanoparticles for targeted cancer therapy and diagnosis. Molecules 26(16):5052
Kim J, Cho H, Lim DK, Joo MK, Kim K (2023) Perspectives for improving the tumor targeting of nanomedicine via the EPR effect in clinical tumors. Int J Mol Sci 24(12):10082
Sharifi M, Cho WC, Ansariesfahani A, Tarharoudi R, Malekisarvar H, Sari S et al (2022) An updated review on EPR-based solid tumor targeting nanocarriers for cancer treatment. Cancers 14(12):2868
Manikkath J, Jishnu PV, Wich PR, Manikkath A, Radhakrishnan R (2022) Nanoparticulate strategies for the delivery of miRNA mimics and inhibitors in anticancer therapy and its potential utility in oral submucous fibrosis. Nanomedicine (Lond) 17(3):181–195
Manikkath J, Parekh HS, Mutalik S (2020) Surface-engineered nanoliposomes with lipidated and non-lipidated peptide-dendrimeric scaffold for efficient transdermal delivery of a therapeutic agent: development, characterization, toxicological and preclinical performance analyses. Eur J Pharm Biopharm 1(156):97–113
Bertrand N, Wu J, Xu X, Kamaly N, Farokhzad OC (2014) Cancer nanotechnology: the impact of passive and active targeting in the era of modern cancer biology. Adv Drug Deliv Rev 66:2–25
Dilliard SA, Siegwart DJ (2023) Passive, active and endogenous organ-targeted lipid and polymer nanoparticles for delivery of genetic drugs. Nat Rev Mater 8(4):282–300
Maeda H (2012) Vascular permeability in cancer and infection as related to macromolecular drug delivery, with emphasis on the EPR effect for tumor-selective drug targeting. Proc Jpn Acad Ser B Phys Biol Sci 9(88):53–71
Park J, Choi Y, Chang H, Um W, Ryu JH, Kwon IC (2019) Alliance with EPR effect: combined strategies to improve the EPR effect in the tumor microenvironment. Theranostics 9(26):8073–8090
Prabhakar U, Maeda H, Jain KR, Sevick-Muraca EM, Zamboni W, Farokhzad OC et al (2013) Challenges and key considerations of the enhanced permeability and retention effect for nanomedicine drug delivery in oncology. Cancer Res 73(8):2412–7
Mathew EN, Berry BC, Yang HW, Carroll RS, Johnson MD (2022) Delivering therapeutics to glioblastoma: overcoming biological constraints. Int J Mol Sci 23(3):1711
Zhang M, Du Y, Wang S, Chen B (2020) A review of biomimetic nanoparticle drug delivery systems based on cell membranes. Drug Design, Dev Ther 14:5495–503
Li YJ, Wu JY, Liu J, Qiu X, Xu W, Tang T et al (2021) From blood to brain: blood cell-based biomimetic drug delivery systems. Drug Deliv 28(1):1214–1225
Yang R, Wei T, Goldberg H, Wang W, Cullion K, Kohane DS (2017) Getting drugs across biological barriers. Adv Mater 29(37):1606596
Liu J, Cabral H, Mi P (2024) Nanocarriers address intracellular barriers for efficient drug delivery, overcoming drug resistance, subcellular targeting and controlled release. Adv Drug Delivery Rev 207:115239
Azizi M, Jahanban-Esfahlan R, Samadian H, Hamidi M, Seidi K, Dolatshahi-Pirouz A, Shahbazi MA (2023) Multifunctional nanostructures: intelligent design to overcome biological barriers. Materials Today Bio 20:100672
Patra JK, Das G, Fraceto LF, Campos EVR, del Rodriguez-Torres MP, Acosta-Torres LS et al (2018) Nano based drug delivery systems: recent developments and future prospects. J Nanobiotechnology 16(1):71
Hu D, Xia M, Wu L, Liu H, Chen Z, Xu H et al (2023) Challenges and advances for glioma therapy based on inorganic nanoparticles. Mater Today Bio 20:100673
Zhang M, Gao S, Yang D, Fang Y, Lin X, Jin X et al (2021) Influencing factors and strategies of enhancing nanoparticles into tumors in vivo. Acta Pharm Sin B 11(8):2265–2285
Lu B, Wang J, Hendriks AJ, Nolte TM (2023) Clearance of nanoparticles from blood: effects of hydrodynamic size and surface coatings. Environ Sci Nano 11(1):406–417
Gupta T, Sahoo RK, Singh H, Katke S, Chaurasiya A, Gupta U (2023) Lipid-based nanocarriers in the treatment of glioblastoma multiforme (GBM): challenges and opportunities. AAPS PharmSciTech 24(4):107
Han X, Gong C, Yang Q, Zheng K, Wang Z, Zhang W (2024) Biomimetic nano-drug delivery system: an emerging platform for promoting tumor treatment. Int J Nanomedicine 19:571–608
Kadry H, Noorani B, Cucullo L (2020) A blood–brain barrier overview on structure, function, impairment, and biomarkers of integrity. Fluids Barriers CNS 17(1):69
Daneman R, Prat A (2015) The blood-brain barrier. Cold Spring Harb Perspect Biol 7(1):a020412
Brown LS, Foster CG, Courtney JM, King NE, Howells DW, Sutherland BA (2019) Pericytes and neurovascular function in the healthy and diseased brain. Front Cell Neurosci 13(June):1–9
Yalamarty SSK, Filipczak N, Li X, Subhan MA, Parveen F, Ataide JA et al (2023) Mechanisms of resistance and current treatment options for glioblastoma multiforme (GBM). Cancers (Basel) 15(7):2116
Marcucci F, Corti A, Ferreri AJM (2021) Breaching the blood–brain tumor barrier for tumor therapy. Cancers 13(10):2391
Onishi M, Kurozumi K, Ichikawa T, Date I (2013) Mechanisms of tumor development and anti-angiogenic therapy in glioblastoma multiforme. Neurol Med Chir (Tokyo) 53(11):755–763
Dubois LG, Campanati L, Righy C, D’Andrea-Meira I, Spohr TCL de S e, Porto-Carreiro I et al (2014) Gliomas and the vascular fragility of the blood-brain barrier. Front Cell Neurosci 8:418
Qiu Z, Yu Z, Xu T, Wang L, Meng N, Jin H, Xu B (2022) Novel nano-drug delivery system for brain tumor treatment. Cells 11(23):3761
Mi P, Cabral H, Kataoka K (2020) Ligand-installed nanocarriers toward precision therapy, vol 32. Wiley-VCH Verlag, Advanced Materials
Lin YJ, Wei KC, Chen PY, Lim M, Hwang TL (2021) Roles of neutrophils in glioma and brain metastases. Front Immunol 12(August):1–19
Xue J, Zhao Z, Zhang L, Xue L, Shen S, Wen Y et al (2017) Neutrophil-mediated anticancer drug delivery for suppression of postoperative malignant glioma recurrence. Nat Nanotechnol 12(7):692–700
Li J, Zeng H, Li L, Song M, Dong M (2023) Biomembrane-wrapped gene delivery nanoparticles for cancer therapy. Front Bioeng Biotechnol 11(June):1–17
Fu S, Liang M, Wang Y, Cui L, Gao C, Chu X et al (2019) Dual-modified novel biomimetic nanocarriers improve targeting and therapeutic efficacy in glioma. ACS Appl Mater Interfaces 11(2):1841–1854
Liu Y, Luo J, Chen X, Liu W, Chen T (2019) Cell membrane coating technology: a promising strategy for biomedical applications. Nano-Micro Lett 11:1–46. https://doi.org/10.1007/s40820-019-0330-9
Gerstner ER, Batchelor TT (2012) Antiangiogenic therapy for glioblastoma. Cancer J 18(1):45–50
Zhao Z, Wang D, Li Y (2023) Versatile biomimetic nanomedicine for treating cancer and inflammation disease. Med Rev 3(2):123–51
Madhankumar AB, Mrowczynski OD, Patel SR, Weston CL, Zacharia BE, Glantz MJ et al (2017) Interleukin-13 conjugated quantum dots for identification of glioma initiating cells and their extracellular vesicles. Acta Biomater 58:205–213
Zein R, Sharrouf W, Selting K (2020) Physical properties of nanoparticles that result in improved cancer targeting. Journal of Oncology 2020(1):5194780
Yuan BO, Zhao Y, Dong S, Sun Y, Hao F, Xie J et al (2019) Cell-penetrating peptide-coated liposomes for drug delivery across the blood-brain barrier. Anticancer Res 39(1):237–243
Kreuter J, Alyautdin RN, Kharkevich DA, Ivanov AA (1995) Passage of peptides through the blood-brain barrier with colloidal polymer particles (nanoparticles). Brain Res 674(1):171–174
Mendanha D, Vieira de Castro J, Ferreira H, Neves NM (2021) Biomimetic and cell-based nanocarriers – new strategies for brain tumor targeting. J Controlled Release 337(March):482–93
Al-Hetty HRAK, Kadhim MS, Al-Tamimi JHZ, Ahmed NM, Jalil AT, Saleh MM et al (2023) Implications of biomimetic nanocarriers in targeted drug delivery. Emergent Mater 6(1):1–13
Robey RW, Pluchino KM, Hall MD, Fojo AT, Bates SE, Gottesman MM (2018) Revisiting the role of ABC transporters in multidrug-resistant cancer. Nat Rev Cancer 18(7):452–464
Dong Y, Liao H, Yu J, Fu H, Zhao D, Gong K et al (2019) Incorporation of drug efflux inhibitor and chemotherapeutic agent into an inorganic/organic platform for the effective treatment of multidrug resistant breast cancer. J Nanobiotechnology 17(1):125
Wang T, Zhang H, Qiu W, Han Y, Liu H, Li Z (2022) Biomimetic nanoparticles directly remodel immunosuppressive microenvironment for boosting glioblastoma immunotherapy. Bioact Mater 16:418–432
Al-Hetty HRAK, Kadhim MS, Al-Tamimi JHZ, Ahmed NM, Jalil AT, Saleh MM et al (2023) Implications of biomimetic nanocarriers in targeted drug delivery. Emergent Mater 6(1):1–13
Yao Y, Zhou Y, Liu L, Xu Y, Chen Q, Wang Y et al (2020) Nanoparticle-based drug delivery in cancer therapy and its role in overcoming drug resistance. Front Mol Biosci 7(August):1–14
Manikkath J, Sumathy TK, Manikkath A, Mutalik S (2018) Delving deeper into dermal and transdermal drug delivery: factors and mechanisms associated with nanocarrier-mediated strategies. Curr Pharm Des 24(27):3210–3222. https://doi.org/10.2174/1381612824666180924122640. (PMID: 30246632)
Danhier F, Feron O, Préat V (2010) To exploit the tumor microenvironment: passive and active tumor targeting of nanocarriers for anti-cancer drug delivery. J Control Release 148(2):135–146
Manikkath J, Subramony JA (2021) Toward closed-loop drug delivery: integrating wearable technologies with transdermal drug delivery systems. Adv Drug Deliv Rev 179:113997
Katayama Y, Uchino J, Chihara Y, Tamiya N, Kaneko Y, Yamada T et al (2019) Tumor neovascularization and developments in therapeutics. Cancers 11(3):316
Shinde VR, Revi N, Murugappan S, Singh SP, Rengan AK (2022) Enhanced permeability and retention effect: a key facilitator for solid tumor targeting by nanoparticles. Photodiagnosis Photodyn Ther. 39:102915. https://www.sciencedirect.com/science/article/pii/S1572100022002010. Accessed 24 Apr 2024
Elumalai K, Srinivasan S, Shanmugam A (2023) Review of the efficacy of nanoparticle-based drug delivery systems for cancer treatment. Biomed Technol 2024(5):109–22. https://doi.org/10.1016/j.bmt.2023.09.001
Attia MF, Anton N, Wallyn J, Omran Z, Vandamme TF (2019) An overview of active and passive targeting strategies to improve the nanocarriers efficiency to tumour sites. J Pharm Pharmacol 71(8):1185–1198
Guido C, Maiorano G, Cortese B, Amone SD, Elena I (2020) Biomimetic nanocarriers for cancer target therapy. Nanomaterials 10(15):2955
Malachowski T, Hassel A (2020) Engineering nanoparticles to overcome immunological barriers for enhanced drug delivery. Engineered Regeneration 1:35–50. https://www.sciencedirect.com/science/article/pii/S2666138120300049. Accessed 24 Apr 2024
Guo C, Yuan H, Wang Y, Feng Y, Zhang Y, Yin T et al (2023) The interplay between PEGylated nanoparticles and blood immune system. Adv Drug Deliv Rev 200:115044. https://www.sciencedirect.com/science/article/pii/S0169409X23003599. Accessed 24 Apr 2024
Hu CMJ, Zhang L, Aryal S, Cheung C, Fang RH, Zhang L (2011) Erythrocyte membrane-camouflaged polymeric nanoparticles as a biomimetic delivery platform. Proc Natl Acad Sci U S A 108(27):10980–10985
Liu M, Chu Y, Liu H, Su Y, Zhang Q, Jiao J et al (2020) Accelerated blood clearance of nanoemulsions modified with PEG-cholesterol and PEG-phospholipid derivatives in rats: the effect of PEG-lipid linkages and PEG molecular weights. Mol Pharm 17(4):1059–70. https://doi.org/10.1021/acs.molpharmaceut.9b00770
Khatoon N, Zhang Z, Zhou C, Chu M (2022) Macrophage membrane-coated nanoparticles: a biomimetic approach for enhanced and targeted delivery. Biomater Sci 10(5):1193–1208
Fukuta T, Kogure K (2022) Biomimetic nanoparticle drug delivery systems to overcome biological barriers for therapeutic applications. Chem Pharm Bull (Tokyo) 70(5):334–340
Chugh V, Vijaya Krishna K, Pandit A (2021) Cell membrane-coated mimics: a methodological approach for fabrication, characterization for therapeutic applications, and challenges for clinical translation. ACS Nano 15(11):17080–123. https://doi.org/10.1021/acsnano.1c03800
Mougenot MF, Pereira VS, Costa AL, Lancellotti M, Porcionatto MA, da Silveira JC et al (2022) Biomimetic nanovesicles—sources, design, production methods, and applications. Pharmaceutics 14(8):1761
Carmona-Ribeiro AM (2012) Preparation and characterization of biomimetic nanoparticles for drug delivery. Methods Mol Biol 906:283–294
Wijesinghe U, Thiripuranathar G, Iqbal H, Menaa F (2021) Biomimetic synthesis, characterization, and evaluation of fluorescence resonance energy transfer, photoluminescence, and photocatalytic activity of zinc oxide nanoparticles. Sustainability 13(4):2122
Zhang H, Dong S, Li Z, Feng X, Xu W, Tulinao CMS et al (2020) Biointerface engineering nanoplatforms for cancer-targeted drug delivery. Asian J Pharm Sci 15(4):397–415. https://doi.org/10.1016/j.ajps.2019.11.004
Tang L, He S, Yin Y, Liu H, Hu J, Cheng J et al (2021) Combination of nanomaterials in cell-based drug delivery systems for cancer treatment. Pharmaceutics 13(11):1888
Zhao Z, Wang D (2023) Li Y Versatile biomimetic nanomedicine for treating cancer and inflammation disease. Med Rev 3(2):123–51
Parodi A, Kostyushev D, Brezgin S, Kostyusheva A, Borodina T, Akasov R et al (2022) Biomimetic approaches for targeting tumor-promoting inflammation. Seminars Cancer Biol Academic Press 86:555–67
Li L, Wang J, Kong H, Zeng Y, Liu G (2018) Functional biomimetic nanoparticles for drug delivery and theranostic applications in cancer treatment. Sci Technol Adv Mater 19(1):771–90. https://doi.org/10.1080/14686996.2018.1528850
Zarrin A, Foroozesh M, Hamidi M (2014) Carrier erythrocytes: recent advances, present status, current trends and future horizons. Expert Opin Drug Deliv 11(3):433–447
Piao JG, Wang L, Gao F, You YZ, Xiong Y, Yang L (2014) Erythrocyte membrane is an alternative coating to polyethylene glycol for prolonging the circulation lifetime of gold nanocages for photothermal therapy. ACS Nano 8(10):10414–10425
Chen L, Hong W, Ren W, Xu T, Qian Z, He Z (2021) Recent progress in targeted delivery vectors based on biomimetic nanoparticles. Signal Transduct Target Ther 6(1) https://doi.org/10.1038/s41392-021-00631-2
Chai Z, Hu X, Wei X, Zhan C, Lu L, Jiang K et al (2017) A facile approach to functionalizing cell membrane-coated nanoparticles with neurotoxin-derived peptide for brain-targeted drug delivery. Journal of Controlled Release 264:102–11. https://www.sciencedirect.com/science/article/pii/S0168365917307940. Accessed 24 Apr 2024
Zou Y, Liu Y, Yang Z, Zhang D, Lu Y, Zheng M et al (2018) Effective and targeted human orthotopic glioblastoma xenograft therapy via a multifunctional biomimetic nanomedicine. Adv Mater 30(51):e1803717
Fu S, Liang M, Wang Y, Cui L, Gao C, Chu X et al (2019) Dual-modified novel biomimetic nanocarriers improve targeting and therapeutic efficacy in glioma. ACS Appl Mater Interfaces 11(2):1841–54. https://doi.org/10.1021/acsami.8b18664
Li YJ, Wu JY, Liu J, Qiu X, Xu W, Tang T et al (2021) From blood to brain: blood cell-based biomimetic drug delivery systems. Drug Deliv 28(1):1214–1225
Gao X, Li S, Ding F, Liu X, Wu Y, Li J et al (2021) A virus-mimicking nucleic acid nanogel reprograms microglia and macrophages for glioblastoma therapy. Adv Mater 33(9):e2006116
Wang J, Ma X, Wu Z, Cui B, Zou C, Zhang P et al (2024) Microfluidics-prepared ultra-small biomimetic nanovesicles for brain tumor targeting. Adv Healthc Mater 13(5):220
Li J, Wei Y, Zhang C, Bi R, Qiu Y, Li Y et al (2023) Cell-membrane-coated nanoparticles for targeted drug delivery to the brain for the treatment of neurological diseases. Pharmaceutics 15(2):621
Pang L, Qin J, Han L, Zhao W, Liang J, Xie Z et al (2016) Exploiting macrophages as targeted carrier to guide nanoparticles into glioma. Oncotarget 7(24):37081–37091
Yin T, Fan Q, Hu F, Ma X, Yin Y, Wang B et al (2022) Engineered macrophage-membrane-coated nanoparticles with enhanced PD-1 expression induce immunomodulation for a synergistic and targeted antiglioblastoma activity. Nano Lett 22(16):6606–14. https://doi.org/10.1021/acs.nanolett.2c01863
Liu Y, Zou Y, Feng C, Lee A, Yin J, Chung R et al (2020) Charge conversional biomimetic nanocomplexes as a multifunctional platform for boosting orthotopic glioblastoma RNAi therapy. Nano Lett 20(3):1637–46. https://doi.org/10.1021/acs.nanolett.9b04683
Lin YJ, Wei KC, Chen PY, Lim M, Hwang TL (2021) Roles of neutrophils in glioma and brain metastases. Front Immunol 12(August):1–19
Liu WL, Zou MZ, Qin SY, Cheng YJ, Ma YH, Sun YX et al (2020) Recent advances of cell membrane-coated nanomaterials for biomedical applications. Adv Funct Mater 30(39):1–23
Gupta T, Sahoo RK, Singh H, Katke S, Chaurasiya A, Gupta U (2023) Lipid-based nanocarriers in the treatment of glioblastoma multiforme (GBM): challenges and opportunities. AAPS PharmSciTech 24(4). https://doi.org/10.1208/s12249-023-02555-2
Li Q, Shen J, Wu L, Lei S, Yang Y, Xu W et al (2023) Functional targeted therapy for glioma based on platelet membrane-coated nanogels. Cancer Nanotechnol 14(1):1–17. https://doi.org/10.1186/s12645-023-00167-w
Bhandarkar S, Prabhakar B, Shende P (2021) Quercetin-loaded platelets as a potential targeted therapy for glioblastoma multiforme cell line U373-MG. Biotechnol J 16(12):1–9
Harris JC, Scully MA, Day ES (2019) Cancer cell membrane-coated nanoparticles for cancer management. Cancers (Basel) 11(12):1836
Han X, Gong C, Yang Q, Zheng K, Wang Z, Zhang W (2024) Biomimetic nano-drug delivery system: an emerging platform for promoting tumor treatment. Int J Nanomed 19:571–608
Qiu Z, Yu Z, Xu T, Wang L, Meng N, Jin H et al (2022) Novel nano-drug delivery system for brain tumor treatment. Cells 11(23):3787
Li J, Zeng H, Li L, Song M, Dong M (2023) Biomembrane-wrapped gene delivery nanoparticles for cancer therapy. Front Bioeng Biotechnol 11(June):1–17
Huang X, Hui H, Shang W, Gao P, Zhou Y, Pang W et al (2023) Deep penetrating and sensitive targeted magnetic particle imaging and photothermal therapy of early-stage glioblastoma based on a biomimetic nanoplatform. Adv Sci 10(19):2300544
De Pasquale D, Marino A, Tapeinos C, Pucci C, Rocchiccioli S, Michelucci E et al (2020) Homotypic targeting and drug delivery in glioblastoma cells through cell membrane-coated boron nitride nanotubes. Mater Des. 192:108742. https://www.sciencedirect.com/science/article/pii/S0264127520302768. Accessed 26 Apr 2024
Tapeinos C, Tomatis F, Battaglini M, Larrañaga A, Marino A, Telleria IA et al (2019) Cell membrane-coated magnetic nanocubes with a homotypic targeting ability increase intracellular temperature due to ros scavenging and act as a versatile theranostic system for glioblastoma multiforme. Adv Healthc Mater 8(18):1900782
Mi P (2020) Stimuli-responsive nanocarriers for drug delivery, tumor imaging, therapy and theranostics. Theranostics 10:4557–88
Zou Y, Wang Y, Xu S, Liu Y, Yin J, Lovejoy DB et al (2022) Brain co-delivery of temozolomide and cisplatin for combinatorial glioblastoma chemotherapy. Adv Mater 34(33):2203958. https://doi.org/10.1002/adma.202203958
Tapeinos C, Marino A, Battaglini M, Migliorin S, Brescia R, Scarpellini A et al (2019) Stimuli-responsive lipid-based magnetic nanovectors increase apoptosis in glioblastoma cells through synergic intracellular hyperthermia and chemotherapy. Nanoscale 11(1):72–88. https://doi.org/10.1039/C8NR05520C
Calinescu AA, Kauss MC, Sultan Z, Al-Holou WN, O’Shea SK (2021) Stem cells for the treatment of glioblastoma: a 20- year perspective. CNS Oncol 10(2):CNS73
Lai YH, Su CY, Cheng HW, Chu CY, Jeng L Bin, Chiang CS et al (2023) Stem cell–nanomedicine system as a theranostic bio-gadolinium agent for targeted neutron capture cancer therapy. Nat Commun 14(1):285
Ghosh AK, Ghosh A, Das PK (2024) Nanotechnology meets stem cell therapy for treating glioblastomas: a review. ACS Appl Nano Mater 7(3):2430–2460
Wu L, Li Q, Deng J, Shen J, Xu W, Yang W et al (2021) Platelet-tumor cell hybrid membrane-camouflaged nanoparticles for enhancing therapy efficacy in glioma. Int J Nanomed 16:8433–8446
Zou Y, Sun Y, Wang Y, Zhang D, Yang H, Wang X et al (2023) Cancer cell-mitochondria hybrid membrane coated gboxin-loaded nanomedicines for glioblastoma treatment. Nat Commun 14(1):6051
Choi A, Javius-Jones K, Hong S, Park H (2023) Cell-based drug delivery systems with innate homing capability as a novel nanocarrier platform. Int J Nanomedicine 18:509–525
Zhang SS, Li RQ, Chen Z, Wang XY, Dumont AS, Fan X (2024) Immune cells: potential carriers or agents for drug delivery to the central nervous system. Mil Med Res 11(1):19. https://doi.org/10.1186/s40779-024-00521-y
Zhou J, Li L, Jia M, Liao Q, Peng G, Luo G et al (2023) Dendritic cell vaccines improve the glioma microenvironment: influence, challenges, and future directions. Cancer Med 12(6):7207–7221
Li TF, Li K, Zhang Q, Wang C, Yue Y, Chen Z et al (2018) Dendritic cell-mediated delivery of doxorubicin-polyglycerol-nanodiamond composites elicits enhanced anti-cancer immune response in glioblastoma. Biomaterials 181:35–52. https://www.sciencedirect.com/science/article/pii/S0142961218305209. Accessed 27 Apr 2024
Mooney R, Roma L, Zhao D, Van Haute D, Garcia E, Kim SU et al (2014) Neural stem cell-mediated intratumoral delivery of gold nanorods improves photothermal therapy. ACS Nano 8(12):12450–60. https://doi.org/10.1021/nn505147w
Huyan T, Li H, Peng H, Chen J, Yang R, Zhang W et al (2020) Extracellular vesicles - advanced nanocarriers in cancer therapy: progress and achievements. Int J Nanomedicine 15:6485–6502
Wang X, Zhao X, Zhong Y, Shen J, An W (2022) Biomimetic exosomes: a new generation of drug delivery system. Biomolecules 10(5):1–16
Singh G, Mehra A, Arora S, Gugulothu D, Vora LK, Prasad R et al (2024) Exosome-mediated delivery and regulation in neurological disease progression. Int J Biol Macromol 264:130728. https://www.sciencedirect.com/science/article/pii/S0141813024015320
Araujo-Abad S, Manresa-Manresa A, Rodríguez-Cañas E, Fuentes-Baile M, García-Morales P, Mallavia R et al (2023) Glioblastoma-derived small extracellular vesicles: nanoparticles for glioma treatment. Int J Mol Sci 24(6):5910
Ansari A, Hussain A, Wadekar R, Altamimi MA, Malik A, Mujtaba MA et al (2023) Nanovesicles based drug targeting to control tumor growth and metastasis. Adv Cancer Biol - Metastasis 7:100083. https://www.sciencedirect.com/science/article/pii/S2667394022000570Accessed 27 Apr 2024
Zhu Q, Ling X, Yang Y, Zhang J, Li Q, Niu X et al (2019) Embryonic stem cells-derived exosomes endowed with targeting properties as chemotherapeutics delivery vehicles for glioblastoma therapy. Adv Sci (Weinh) 6(6):1801899
Estevez-ordonez D, Chagoya G, Parr MS, Elsayed GA, Mahavadi AK (2021) Immunovirotherapy for the treatment of glioblastoma and other malignant gliomas. Neurosurg Clin North Am 32(2):265–281
Lam P, Lin RD, Steinmetz NF (2018) Delivery of mitoxantrone using a plant virus-based nanoparticle for the treatment of glioblastomas. J Mater Chem B 6(37):5888–5895
Yang J, Zhang Q, Liu Y, Zhang X, Shan W, Ye S et al (2020) Nanoparticle-based co-delivery of siRNA and paclitaxel for dual-targeting of glioblastoma. Nanomedicine 15(14):1391–409. https://doi.org/10.2217/nnm-2020-0066
Manikkath J, Manikkath A, Lad H, Vora LK, Mudgal J, Shenoy RR, Ashili S, Radhakrishnan R (2023) Nanoparticle-mediated active and passive drug targeting in oral squamous cell carcinoma: current trends and advances. Nanomedicine (Lond) 18(27):2061–2080. https://doi.org/10.2217/nnm-2023-0247
Kadhim ZA, Sulaiman GM, Al-Shammari AM, Khan RA, Al Rugaie O, Mohammed HA (2022) Oncolytic newcastle disease virus co-delivered with modified PLGA nanoparticles encapsulating temozolomide against glioblastoma cells: developing an effective treatment strategy. Molecules 27(12):4078
Finbloom JA, Aanei IL, Bernard JM, Klass SH, Elledge SK, Han K et al (2018) Evaluation of three morphologically distinct virus-like particles as nanocarriers for convection-enhanced drug delivery to glioblastoma. Nanomaterials 8(12):1007
Han S, Lee Y, Lee M (2021) Biomimetic cell membrane-coated DNA nanoparticles for gene delivery to glioblastoma. Journal of Controlled 338:22–32. https://www.sciencedirect.com/science/article/pii/S0168365921004272
Dudu V, Rotari V, Vazquez M (2012) Sendai virus-based liposomes enable targeted cytosolic delivery of nanoparticles in brain tumor-derived cells. J Nanobiotechnol 10(1):9. https://doi.org/10.1186/1477-3155-10-9
Banks WA (2015) Peptides and the blood-brain barrier. Peptides (NY) 72:16–19
Mehrotra S, Kalyan PBG, Nayak PG, Joseph A, Manikkath J (2024) Recent progress in the oral delivery of therapeutic peptides and proteins: overview of pharmaceutical strategies to overcome absorption hurdles. Adv Pharm Bull 14:11–33
Wang R, Yan H, Yu A, Ye L, Zhai G (2021) Cancer targeted biomimetic drug delivery system. J Drug Deliv Sci Technol 63(April):102530. https://doi.org/10.1016/j.jddst.2021.102530
Gregory JV, Kadiyala P, Doherty R, Cadena M, Habeel S, Ruoslahti E et al (2020) Systemic brain tumor delivery of synthetic protein nanoparticles for glioblastoma therapy. Nat Commun 11(1):5687. https://doi.org/10.1038/s41467-020-19225-7
Helal DO, Rouatbi N, Han S, Tzu-Wen Wang J, Walters AA, Abdel-Mottaleb MMA et al (2021) A natural protein based platform for the delivery of temozolomide acid to glioma cells. European Journal of Pharmaceutics and Biopharmaceutics. 169:297–308. https://www.sciencedirect.com/science/article/pii/S0939641121002629. Accessed 24 Apr 2024
Säälik P, Lingasamy P, Toome K, Mastandrea I, Rousso-Noori L, Tobi A et al (2019) Peptide-guided nanoparticles for glioblastoma targeting. Journal of Controlled Release. 308:109–18. https://www.sciencedirect.com/science/article/pii/S0168365919303281. Accessed 24 Apr 2024
Wiwatchaitawee K, Quarterman JC, Geary SM, Salem AK (2021) Enhancement of therapies for glioblastoma (GBM) using nanoparticle-based delivery systems. AAPS PharmSciTech 22(2):71. https://doi.org/10.1208/s12249-021-01928-9
Liu J, Fu M, Wang M, Wan D, Wei Y, Wei X (2022) Cancer vaccines as promising immuno-therapeutics: platforms and current progress. J Hematol Oncol. 15(1):28. https://doi.org/10.1186/s13045-022-01247-x
Hosseinalizadeh H, Rahmati M, Ebrahimi A, O’Connor RS (2023) Current status and challenges of vaccination therapy for glioblastoma. Mol Cancer Ther 22(4):435–446
Blass E, Ott PA (2021) Advances in the development of personalized neoantigen-based therapeutic cancer vaccines. Nat Rev Clin Oncol. 18(4):215–29. https://doi.org/10.1038/s41571-020-00460-2
Zhao T, Li C, Ge H, Lin Y, Kang D (2022) Glioblastoma vaccine tumor therapy research progress. Chin Neurosurg J 8(1):4–8
Lin MJ, Svensson-Arvelund J, Lubitz GS, Marabelle A, Melero I, Brown BD et al (2022) Cancer vaccines: the next immunotherapy frontier. Nat Cancer 3(8):911–26. https://doi.org/10.1038/s43018-022-00418-6
Platten M (2017) EGFRvIII vaccine in glioblastoma-InACT-IVe or not ReACTive enough? Neuro Oncol 19(11):1425–1426
Huang J, Yu J, Tu L, Huang N, Li H, Luo Y (2019) Isocitrate dehydrogenase mutations in glioma: from basic discovery to therapeutics development. Front Oncol 9:506
Zhao Y, Baldin AV, Isayev O, Werner J, Zamyatnin AA Jr, Bazhin AV (2021) Cancer vaccines: antigen selection strategy. Vaccines 9(2):85
Kong Z, Wang Y, Ma W (2018) Vaccination in the immunotherapy of glioblastoma. Hum Vaccin Immunother 14(2):255–268
Cheng Z, Li M, Dey R, Chen Y (2021) Nanomaterials for cancer therapy: current progress and perspectives. J Hematol Oncol 14(1):1–27. https://doi.org/10.1186/s13045-021-01096-0
Datsi A, Sorg RV (2021) Dendritic cell vaccination of glioblastoma: road to success or dead end. Front Immunol 12:770390
Rapp M, Grauer OM, Kamp M, Sevens N, Zotz N, Sabel M et al (2018) A randomized controlled phase II trial of vaccination with lysate-loaded, mature dendritic cells integrated into standard radiochemotherapy of newly diagnosed glioblastoma (GlioVax): study protocol for a randomized controlled trial. Trials 19(1):293
Qin F, Xia F, Chen H, Cui B, Feng Y, Zhang P et al (2021) A guide to nucleic acid vaccines in the prevention and treatment of infectious diseases and cancers: from basic principles to current applications. Front Cell Dev Biol 9:633776
Chehelgerdi M, Chehelgerdi M (2023) The use of RNA-based treatments in the field of cancer immunotherapy. Mol Cancer 22(1):106. https://doi.org/10.1186/s12943-023-01807-w
Zhong H, Liu S, Cao F, Zhao Y, Zhou J, Tang F et al (2021) Dissecting tumor antigens and immune subtypes of glioma to develop mRNA vaccine. Front Immunol 12:709986
Mei Y, Wang X (2023) RNA modification in mRNA cancer vaccines. Clin Exp Med 23(6):1917–31. https://doi.org/10.1007/s10238-023-01020-5
Taiarol L, Formicola B, Magro RD, Sesana S, Re F (2020) An update of nanoparticle-based approaches for glioblastoma multiforme immunotherapy. Nanomedicine 15(19):1861–1871
Vik-Mo EO, Nyakas M, Mikkelsen BV, Moe MC, Due-Tønnesen P, Suso EMI et al (2013) Therapeutic vaccination against autologous cancer stem cells with mRNA-transfected dendritic cells in patients with glioblastoma. Cancer Immunol Immunother 62(9):1499–1509
Lu B, Lim JM, Yu B, Song S, Neeli P, Sobhani N et al (2024) The next-generation DNA vaccine platforms and delivery systems: advances, challenges and prospects. Front Immunol 15:1332939
Wick W, Wick A, Sahm F, Riehl D, von Deimling A, Bendszus M et al (2018) P01. 031 VXM01 phase I study in patients with progressive glioblastoma-final results. Neuro-Oncology 20(3):iii235
Xiong Z, Raphael I, Olin M, Okada H, Li X, Kohanbash G (2024) Glioblastoma vaccines: past, present, and opportunities. EBioMedicine 100:104963. https://doi.org/10.1016/j.ebiom.2023.104963
Coffman RL, Sher A, Seder RA (2010) Vaccine adjuvants: putting innate immunity to work. Immunity 33(4):492–503
Funding
Open access funding provided by Manipal Academy of Higher Education, Manipal. No funding was received to assist with the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
K.N.P. and J.M. wrote the main manuscript text. J.M. and S.M. provided the concept and design of the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Panchal, K.N., Mutalik, S. & Manikkath, J. Biomimetic nanoparticle-driven strategies for targeted drug delivery in glioblastoma. J Nanopart Res 26, 192 (2024). https://doi.org/10.1007/s11051-024-06104-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-024-06104-1