Abstract
Over the past couple of decades, the incidence of breast cancer (BC) has significantly increased among females in comparison to other cancer types. In medicinal terminology, the susceptibility to BC is mainly centered around three hormonal receptors: estrogen (ER), progesterone (PR), and human epidermal growth receptor (HER2). Notably, estrogen- dependent breast cancer has a considerable female demographic, making it treatable with hormonal drugs and less intensive immunotherapy. Conversely, the narrative delves into the ominous type of cancer known as triple-negative breast cancer (TNBC). The orientation of all three receptors falls in a negative direction, which is ineffective for treatments that rely on hormonal or antagonist medicaments. Therefore, the only option available to tackle this type of cancer is chemotherapy, which causes toxicity within the body, is highly expensive, and is non-targeted. To counter this challenge, researchers have pioneered nano-based drug delivery systems (NDDS) owing to their innumerable merits and scientific development. NDDS mainly involves polymeric nanoparticles, liposomes, and dendrimers. This review comprehensively details the advancements in nanoinduced targeted drug delivery systems, with a focus on surface modification techniques for active targeting, enhanced drug release, and improved pharmacokinetics. Critical analysis extends to preclinical and clinical studies, revealing the potential of nano-drug delivery systems in TNBC to surpass traditional therapies, with promising heightened efficacy and reduced side effects.
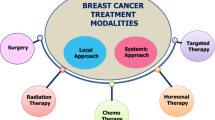
Graphical Abstract














Similar content being viewed by others
Data availability
The paper and supplementary material contain the original contributions that were presented in the study. The appropriate author can be contacted for more information.
Abbreviations
- TNBC :
-
Triple-negative Breast Cancer
- NDDS :
-
Nano-based drug delivery systems
- LAR :
-
Luminal Androgen Receptor
- CSC S :
-
Cancer Stem Cells
- DBD :
-
DNA-binding domain
- EREs :
-
oestrogen response elements
- BRCA1 :
-
Breast Cancer gene 1
- DFS :
-
Disease-Free Survival
- PCR :
-
Polymerase Chain Reaction
- MRI :
-
Magnetic Resonance Imaging
- IHC :
-
Immunohistochemistry
- BCT :
-
Breast conservation therapy
- DDR :
-
DNA Damage Response
- HRD :
-
Homologous recombination deficiency
- PARA :
-
Poly(ADP-ribose) Polymerase
- ORR :
-
Overall response rate
- LOH :
-
Loss of heterozygosity
- CXCR4 :
-
Chemokine (C-X-C motif) Receptor 4
- siRNA :
-
Small interfering RNA
- RSK2 :
-
Ribosomal S6 Kinase 2
- Gpx1 :
-
Glutathione Peroxidase-1
- miRNA :
-
MicroRNA
- PD-1 :
-
Programmed Cell Death Protein 1
- PD-L1 :
-
Programmed Death-Ligand 1
- TILs :
-
Tumor infiltrating lymphocytes
- CBS :
-
Conservative breast surgery
- FDA :
-
Food and Drug Administration
- PEG :
-
Polyethylene Glycol
- SPION :
-
Superparamagnetic Nano particulates
- SLNs :
-
Solid lipid nanomaterials
- RME :
-
Receptor-mediated endocytosis
- PLA :
-
Poly (lactic acid)
- TGIs :
-
Tumor growth inhibition rates
- BMDM :
-
Bone marrow-derived macrophages
- TNF-α :
-
Tumor Necrosis Factor-alpha
- SST :
-
Synthetic somatostatin analogs
- SST :
-
Stands for somatostatin
- SSTR2 :
-
Stands for Somatostatin Receptor Type 2
- AF :
-
Atrial Fibrillation
- NMOFs :
-
Nanostructured metal-organic frameworks
- IRMOF-3 :
-
Iso-reticular metal-organic framework-3
- CAM :
-
Complementary and Alternative Medicine
- ROS :
-
Reactive oxygen species
- MSN :
-
Mesoporous silica nanoparticles
- HUVEC :
-
Human umbilical vein endothelial cells
- FITC :
-
Fluorescein Isothiocyanate
- XFM :
-
X-ray fluorescence microscopy
- LBL :
-
Layer by layer
- BTNPs :
-
Barium titanate nanoparticles
- CLSM :
-
Confocal laser scanning microscopy
- EMT :
-
Epithelial-mesenchymal transition
- NLCs :
-
Nanostructured lipid carriers
- LPH-NPs :
-
Lipid-polymer hybrid nanoparticles
References
Martin C, Aibani N, Callan JF, Callan B (2016) Recent advances in amphiphilic polymers for simultaneous delivery of hydrophobic and hydrophilic drugs. Ther. Deliv 7(1):15–31. https://doi.org/10.4155/tde.15.84
Guan L, Rizzello L, Battaglia G (2015) Polymersomes and their applications in cancer delivery and therapy. Nanomedicine 10(17):2757–2780. https://doi.org/10.2217/nnm.15.110
Karandish F et al (2018) Nucleus-Targeted, Echogenic Polymersomes for Delivering a Cancer Stemness Inhibitor to Pancreatic Cancer Cells. Biomacromolecules 19(10):4122–4132. https://doi.org/10.1021/acs.biomac.8b01133
Golombek SK et al (2018) Tumor targeting via EPR: Strategies to enhance patient responses. Adv Drug Deliv Rev 130:17–38. https://doi.org/10.1016/j.addr.2018.07.007
MacDonald I, Nixon NA, Khan OF (2022) Triple-Negative Breast Cancer: A Review of Current Curative Intent Therapies. Curr Oncol 29(7):4768–4778. https://doi.org/10.3390/curroncol29070378
Kesharwani P, Sheikh A, Abourehab MAS, Salve R, Gajbhiye V (2023) A combinatorial delivery of survivin targeted siRNA using cancer selective nanoparticles for triple negative breast cancer therapy. J Drug Deliv Sci Technol 80:104164. https://doi.org/10.1016/j.jddst.2023.104164
Kulkarni P, Haldar MK, You S, Choi Y, Mallik S (2016) Hypoxia-Responsive Polymersomes for Drug Delivery to Hypoxic Pancreatic Cancer Cells. Biomacromolecules 17(8):2507–2513. https://doi.org/10.1021/acs.biomac.6b00350
Confeld MI et al (2020) Targeting the Tumor Core: Hypoxia-Responsive Nanoparticles for the Delivery of Chemotherapy to Pancreatic Tumors. Mol Pharm 17(8):2849–2863. https://doi.org/10.1021/acs.molpharmaceut.0c00247
Deng ZJ, Morton SW, Ben-Akiva E, Dreaden EC, Shopsowitz KE, Hammond PT (2013) Layer-by-Layer Nanoparticles for Systemic Codelivery of an Anticancer Drug and siRNA for Potential Triple-Negative Breast Cancer Treatment. ACS Nano 7(11):9571–9584. https://doi.org/10.1021/nn4047925
Zhang Y et al (2017) Tumor-Targeting Micelles Based on Linear-Dendritic PEG–PTX 8 Conjugate for Triple Negative Breast Cancer Therapy. Mol Pharm 14(10):3409–3421. https://doi.org/10.1021/acs.molpharmaceut.7b00430
Brinkman AM et al (2016) Aminoflavone-loaded EGFR-targeted unimolecular micelle nanoparticles exhibit anti-cancer effects in triple negative breast cancer. Biomaterials 101:20–31. https://doi.org/10.1016/j.biomaterials.2016.05.041
Torres-Pérez SA, del P Ramos-Godínez M, Ramón-Gallegos E (2019) Effect of methotrexate conjugated PAMAM dendrimers on the viability of breast cancer cells. p 050014. https://doi.org/10.1063/1.5095929
Wu X, Han Z, Schur RM, Lu Z-R (2016) Targeted Mesoporous Silica Nanoparticles Delivering Arsenic Trioxide with Environment Sensitive Drug Release for Effective Treatment of Triple Negative Breast Cancer. ACS Biomater Sci Eng 2(4):501–507. https://doi.org/10.1021/acsbiomaterials.5b00398
Lü L et al (2017) MicroRNAs in the prognosis of triple-negative breast cancer. Medicine (Baltimore) 96(22):e7085. https://doi.org/10.1097/MD.0000000000007085
Zhu H, Dai M, Chen X, Chen X, Qin S, Dai S (2017) Integrated analysis of the potential roles of miRNA-mRNA networks in triple negative breast cancer. Mol Med Rep 16(2):1139–1146. https://doi.org/10.3892/mmr.2017.6750
Rastogi P et al (2008) Preoperative Chemotherapy: Updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27. J Clin Oncol 26(5):778–785. https://doi.org/10.1200/JCO.2007.15.0235
Zhao L, Gu C, Gan Y, Shao L, Chen H, Zhu H (2020) Exosome-mediated siRNA delivery to suppress postoperative breast cancer metastasis. J Control Release 318:1–15. https://doi.org/10.1016/j.jconrel.2019.12.005
Guo P, You J-O, Yang J, Jia D, Moses MA, Auguste DT (2014) Inhibiting Metastatic Breast Cancer Cell Migration via the Synergy of Targeted, pH-triggered siRNA Delivery and Chemokine Axis Blockade. Mol Pharm. 11(3):755–765. https://doi.org/10.1021/mp4004699
Lee E et al (2020) Glutathione peroxidase-1 regulates adhesion and metastasis of triple-negative breast cancer cells via FAK signaling. Redox Biol 29:101391. https://doi.org/10.1016/j.redox.2019.101391
Zhang Y et al (2020) Non-SMC Condensin I Complex Subunit D2 Is a Prognostic Factor in Triple-Negative Breast Cancer for the Ability to Promote Cell Cycle and Enhance Invasion. Am J Pathol. 190(1):37–47. https://doi.org/10.1016/j.ajpath.2019.09.014
Medina MA et al (2020) Triple-Negative Breast Cancer: A Review of Conventional and Advanced Therapeutic Strategies. Int J Environ Res Public Health 17(6):2078. https://doi.org/10.3390/ijerph17062078
Dana H et al (2017) Molecular Mechanisms and Biological Functions of siRNA. IJBS 13
Macias H, Hinck L (2012) Mammary gland development. Wiley Interdiscip Rev Dev Biol 1(4):533–557. https://doi.org/10.1002/wdev.35
Huebner RJ, Ewald AJ (2014) Cellular foundations of mammary tubulogenesis. Semin Cell Dev Biol 31:124–131. https://doi.org/10.1016/j.semcdb.2014.04.019
Hunter T (2000) Signaling—2000 and Beyond. Cell 100(1):113–127. https://doi.org/10.1016/S0092-8674(00)81688-8
Hunter T (2007) The Age of Crosstalk: Phosphorylation, Ubiquitination, and Beyond. Mol Cell 28(5):730–738. https://doi.org/10.1016/j.molcel.2007.11.019
Sever R, Brugge JS (2015) Signal Transduction in Cancer. Cold Spring Harb Perspect Med 5(4):a006098–a006098. https://doi.org/10.1101/cshperspect.a006098
Kumar V, Green S, Stack G, Berry M, Jin J-R, Chambon P (1987) Functional domains of the human estrogen receptor. Cell 51(6):941–951. https://doi.org/10.1016/0092-8674(87)90581-2
Kent Osborne C, Schiff R, Fuqua SAW, Shou J (2001). Estrogen Receptor: Current Understanding of Its Activation and Modulation. Clin Cancer Res 7
Renoir J-M, Marsaud V, Lazennec G (2013) Estrogen receptor signaling as a target for novel breast cancer therapeutics. Biochem Pharmacol 85(4):449–465. https://doi.org/10.1016/j.bcp.2012.10.018
Cheskis BJ, Greger JG, Nagpal S, Freedman LP (2007) Signaling by estrogens. J Cell Physiol 213(3):610–617. https://doi.org/10.1002/jcp.21253
Björnström L, Sjöberg M (2005) Mechanisms of Estrogen Receptor Signaling: Convergence of Genomic and Nongenomic Actions on Target Genes. Mol Endocrinol 19(4):833–842. https://doi.org/10.1210/me.2004-0486
Saha Roy S, Vadlamudi RK (2012) Role of Estrogen Receptor Signaling in Breast Cancer Metastasis. Int J Breast Cancer 2012:1–8. https://doi.org/10.1155/2012/654698
Klinge CM (2000) Estrogen receptor interaction with co-activators and co-repressors☆. Steroids 65(5):227–251. https://doi.org/10.1016/S0039-128X(99)00107-5
Marino M, Galluzzo P, Ascenzi P (2006) Estrogen Signaling Multiple Pathways to Impact Gene Transcription. Curr Genomics 7(8):497–508. https://doi.org/10.2174/138920206779315737
Fan S et al (1999) BRCA1 Inhibition of Estrogen Receptor Signaling in Transfected Cells. Science (80-) 284(5418):1354–1356 https://doi.org/10.1126/science.284.5418.1354.
Nadji M, Gomez-Fernandez C, Ganjei-Azar P, Morales AR (2005) Immunohistochemistry of Estrogen and Progesterone Receptors Reconsidered. Am J Clin Pathol 123(1):21–27. https://doi.org/10.1309/4WV79N2GHJ3X1841
Medina MA, Oza G, Sharma A, Arriaga LG, Hernández Hernández JM, Rotello VM, Ramirez JT (2020) Triple-Negative Breast Cancer: A Review of Conventional and Advanced Therapeutic Strategies. https://doi.org/10.3390/ijerph17062078.
Shokooh MK, Emami F, Jeong J-H, Yook S Bio-Inspired and Smart Nanoparticles for Triple Negative Breast Cancer Microenvironment. Pharmaceutics 13:287. https://doi.org/10.3390/pharmaceutics13020287
Silva-Cázares MB, Saavedra-Leos MZ, Jordan-Alejandre E, Nuñez-Olvera S, Cómpean-Martínez I, López-Camarillo C (2020) Lipid-based nanoparticles for the therapeutic delivery of non-coding RNAs in breast cancer (Review). Oncol Rep 44:2353-2363 https://doi.org/10.3892/or.2020.7791
Bertrand N, Wu J, Xu X, Kamaly N, Farokhzad OC (2014) Cancer nanotechnology: The impact of passive and active targeting in the era of modern cancer biology. Adv Drug Deliv Rev 66:2–25. https://doi.org/10.1016/j.addr.2013.11.009
Miller-Kleinhenz JM, Bozeman EN, Yang L (2015) Targeted nanoparticles for image-guided treatment of triple-negative breast cancer: clinical significance and technological advances. Wiley Interdiscip Rev Nanomedicine Nanobiotechnology 7(6):797–816. https://doi.org/10.1002/wnan.1343
Thun MJ, Henley SJ, Patrono C (2002) Nonsteroidal Anti-inflammatory Drugs as Anticancer Agents: Mechanistic, Pharmacologic, and Clinical Issues. JNCI J Natl Cancer Inst 94(4):252–266. https://doi.org/10.1093/jnci/94.4.252
Ramirez LY, Huestis SE, Yap TY, Zyzanski S, Drotar D, Kodish E (2009) Potential chemotherapy side effects: What do oncologists tell parents? Pediatr Blood Cancer 52(4):497–502. https://doi.org/10.1002/pbc.21835
Bagnyukova TV, Serebriiskii IG, Zhou Y, Hopper-Borge EA, Golemis EA, Astsaturov I (2010) Chemotherapy and signaling. Cancer Biol Ther 10(9):839–853. https://doi.org/10.4161/cbt.10.9.13738
Hu D et al (2018) Oxygen-generating Hybrid Polymeric Nanoparticles with Encapsulated Doxorubicin and Chlorin e6 for Trimodal Imaging-Guided Combined Chemo-Photodynamic Therapy. Theranostics 8(6):1558–1574. https://doi.org/10.7150/thno.22989
Yuan J-D et al (2018) pH-sensitive polymeric nanoparticles of mPEG-PLGA-PGlu with hybrid core for simultaneous encapsulation of curcumin and doxorubicin to kill the heterogeneous tumour cells in breast cancer. Artif Cells, Nanomedicine, Biotechnol 46(sup1):302–313. https://doi.org/10.1080/21691401.2017.1423495
Khanna V, Kalscheuer S, Kirtane A, Zhang W, Panyam J (2019) Perlecan-targeted nanoparticles for drug delivery to triple-negative breast cancer. Futur Drug Discov 1(1) https://doi.org/10.4155/fdd-2019-0005
Zhang R et al (2019) Immune Checkpoint Blockade Mediated by a Small-Molecule Nanoinhibitor Targeting the PD-1/PD-L1 Pathway Synergizes with Photodynamic Therapy to Elicit Antitumor Immunity and Antimetastatic Effects on Breast Cancer. Small 15(49):1903881. https://doi.org/10.1002/smll.201903881
Wang S et al (2017) Co-delivery of gambogic acid and TRAIL plasmid by hyaluronic acid grafted PEI-PLGA nanoparticles for the treatment of triple negative breast cancer. Drug Deliv 24(1):1791–1800. https://doi.org/10.1080/10717544.2017.1406558
Duan X, Chan C, Guo N, Han W, Weichselbaum RR, Lin W (2016) Photodynamic Therapy Mediated by Nontoxic Core-Shell Nanoparticles Synergizes with Immune Checkpoint Blockade To Elicit Antitumor Immunity and Antimetastatic Effect on Breast Cancer. J Am Chem Soc 138(51):16686–16695. https://doi.org/10.1021/jacs.6b09538
Martin JD, Cabral H, Stylianopoulos T, Jain RK (2020) Improving cancer immunotherapy using nanomedicines: progress, opportunities and challenges. Nat Rev Clin Oncol 17(4):251–266. https://doi.org/10.1038/s41571-019-0308-z
Goldberg MS (2019) Improving cancer immunotherapy through nanotechnology. Nat Rev Cancer 19(10):587–602. https://doi.org/10.1038/s41568-019-0186-9
Schmid P et al (2018) Atezolizumab and Nab-Paclitaxel in Advanced Triple-Negative Breast Cancer. N Engl J Med 379(22):2108–2121. https://doi.org/10.1056/NEJMoa1809615
Thambi T et al (2014) Hypoxia-responsive polymeric nanoparticles for tumor-targeted drug delivery. Biomaterials 35(5):1735–1743. https://doi.org/10.1016/j.biomaterials.2013.11.022
Esfandiari N, Arzanani MK, Soleimani M, Kohi-Habibi M, Svendsen WE (2016) A new application of plant virus nanoparticles as drug delivery in breast cancer. Tumor Biol 37(1):1229–1236. https://doi.org/10.1007/s13277-015-3867-3
Le DHT, Lee KL, Shukla S, Commandeur U, Steinmetz NF (2017) Potato virus X, a filamentous plant viral nanoparticle for doxorubicin delivery in cancer therapy. Nanoscale 9(6):2348–2357. https://doi.org/10.1039/C6NR09099K
Wahajuddin and Arora (2012) Superparamagnetic iron oxide nanoparticles: magnetic nanoplatforms as drug carriers. Int J Nanomedicine 3445. https://doi.org/10.2147/IJN.S30320
Neuberger T, Schöpf B, Hofmann H, Hofmann M, von Rechenberg B (2005) Superparamagnetic nanoparticles for biomedical applications: Possibilities and limitations of a new drug delivery system. J Magn Magn Mater 293(1):483–496. https://doi.org/10.1016/j.jmmm.2005.01.064
Trivino A, Gumireddy A, Chauhan H (2019) Drug-Lipid-Surfactant Miscibility for the Development of Solid Lipid Nanoparticles. AAPS PharmSciTech 20(2):46. https://doi.org/10.1208/s12249-018-1229-3
Meng H et al (2013) Codelivery of an Optimal Drug/siRNA Combination Using Mesoporous Silica Nanoparticles To Overcome Drug Resistance in Breast Cancer in Vitro and in Vivo. ACS Nano 7(2):994–1005. https://doi.org/10.1021/nn3044066
Yong K-T et al (2007) Quantum Rod Bioconjugates as Targeted Probes for Confocal and Two-Photon Fluorescence Imaging of Cancer Cells. Nano Lett 7(3):761–765. https://doi.org/10.1021/nl063031m
Loo C, Lowery A, Halas N, West J, Drezek R (2005) Immunotargeted Nanoshells for Integrated Cancer Imaging and Therapy. Nano Lett 5(4):709–711. https://doi.org/10.1021/nl050127s
Hirsch LR et al (2003) Nanoshell-mediated near-infrared thermal therapy of tumors under magnetic resonance guidance. Proc Natl Acad Sci 100(23):13549–13554. https://doi.org/10.1073/pnas.2232479100
Pan B et al (2007) Dendrimer-Modified Magnetic Nanoparticles Enhance Efficiency of Gene Delivery System. Cancer Res. 67(17):8156–8163. https://doi.org/10.1158/0008-5472.CAN-06-4762
Cloninger MJ (2002) Biological applications of dendrimers. Curr Opin Chem Biol 6(6):742–748. https://doi.org/10.1016/S1367-5931(02)00400-3
Toh T-B et al (2014) Nanodiamond–Mitoxantrone Complexes Enhance Drug Retention in Chemoresistant Breast Cancer Cells. Mol Pharm 11(8):2683–2691. https://doi.org/10.1021/mp5001108
Zhang P et al (2016) Better pathologic complete response and relapse-free survival after carboplatin plus paclitaxel compared with epirubicin plus paclitaxel as neoadjuvant chemotherapy for locally advanced triple-negative breast cancer: a randomized phase 2 trial. Oncotarget 7(37):60647–60656 https://doi.org/10.18632/oncotarget.10607.
Al-Mahmood S, Sapiezynski J, Garbuzenko OB, Minko T (2018) Metastatic and triple-negative breast cancer: challenges and treatment options. Drug Deliv Transl Res 8(5):1483–1507. https://doi.org/10.1007/s13346-018-0551-3
Piccart-Gebhart MJ et al (2005) Trastuzumab after Adjuvant Chemotherapy in HER2-Positive Breast Cancer. N Engl J Med 353(16):1659–1672. https://doi.org/10.1056/NEJMoa052306
Waks AG, Winer EP (2019) Breast Cancer Treatment. JAMA 321(3):288. https://doi.org/10.1001/jama.2018.19323
Lim E, Palmieri C, Tilley WD (2016) Renewed interest in the progesterone receptor in breast cancer. Br J Cancer 115(8):909–911. https://doi.org/10.1038/bjc.2016.303
Joshi H, Press MF (2018) Molecular Oncology of Breast Cancer. In: The Breast, Elsevier, pp. 282-307.e5. https://doi.org/10.1016/B978-0-323-35955-9.00022-2
Al-Warhi T, Sabt A, Elkaeed EB, Eldehna WM (2020) Recent advancements of coumarin-based anticancer agents: An up-to-date review. Bioorg Chem 103:104163. https://doi.org/10.1016/j.bioorg.2020.104163
Bae YH, Park K (2011) Targeted drug delivery to tumors: Myths, reality and possibility. J Control Release 153(3):198–205. https://doi.org/10.1016/j.jconrel.2011.06.001
Zhang L, Gu F, Chan J, Wang A, Langer R, Farokhzad O (2008) Nanoparticles in Medicine: Therapeutic Applications and Developments. Clin Pharmacol Ther 83(5):761–769. https://doi.org/10.1038/sj.clpt.6100400
Hirsjarvi S, Passirani C, Benoit J-P (2011) Passive and Active Tumour Targeting with Nanocarriers. Curr Drug Discov Technol 8(3):188–196. https://doi.org/10.2174/157016311796798991
Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, Langer R (2007) Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol
Mostafavi Yazdi SJ, Cho S, Lee J-H (2021) Analysis and optimization of six types of two-coil inductive for the human implantable wireless electrocardiogram sensor. In: Optical Fibers and Sensors for Medical Diagnostics. Treatment and Environ Appl XXI, p 12. https://doi.org/10.1117/12.2582372
Bianchini G, Balko JM, Mayer IA, Sanders ME, Gianni L (2016) Triple-negative breast cancer: challenges and opportunities of a heterogeneous disease. Nat Rev Clin Oncol 13(11):674–690. https://doi.org/10.1038/nrclinonc.2016.66
Bosch A, Eroles P, Zaragoza R, Viña JR, Lluch A (2010) Triple-negative breast cancer: Molecular features, pathogenesis, treatment and current lines of research. Cancer Treat Rev 36(3):206–215. https://doi.org/10.1016/j.ctrv.2009.12.002
Murugan C et al (2016) Combinatorial nanocarrier based drug delivery approach for amalgamation of anti-tumor agents in breast cancer cells: an improved nanomedicine strategy. Sci Rep 6(1):34053. https://doi.org/10.1038/srep34053
Pawar A, Prabhu P (2019) Nanosoldiers: A promising strategy to combat triple negative breast cancer. Biomed Pharmacother 110:319–341. https://doi.org/10.1016/j.biopha.2018.11.122
Abdulkarim BS, Cuartero J, Hanson J, Deschênes J, Lesniak D, Sabri S (2011) Increased Risk of Locoregional Recurrence for Women With T1–2N0 Triple-Negative Breast Cancer Treated With Modified Radical Mastectomy Without Adjuvant Radiation Therapy Compared With Breast-Conserving Therapy. J Clin Oncol. 29(21):2852–2858. https://doi.org/10.1200/JCO.2010.33.4714
von Minckwitz G et al (2010) Abstract S4-6: Neoadjuvant Chemotherapy with or without Bevacizumab: Primary Efficacy Endpoint Analysis of the GEPARQUINTO Study (GBG 44). Cancer Res 70(24_Supplement): S4-6 https://doi.org/10.1158/0008-5472.SABCS10-S4-6
Gerber B et al (2013) Neoadjuvant bevacizumab and anthracycline–taxane-based chemotherapy in 678 triple-negative primary breast cancers; results from the geparquinto study (GBG 44). Ann Oncol 24(12):2978–2984. https://doi.org/10.1093/annonc/mdt361
Gennari A et al (2008) HER2 Status and Efficacy of Adjuvant Anthracyclines in Early Breast Cancer: A Pooled Analysis of Randomized Trials. JNCI J Natl Cancer Inst 100(1):14–20. https://doi.org/10.1093/jnci/djm252
Slamon D, Mackey J, Robert N, Crown J, Martin M, Eiremann W et al (2007) Role of anthracycline-based therapy in the adjuvant treatment of breast cancer: efficacy analyses determined by molecular subtypes of the disease. Springer
von Minckwitz G et al (2012) Definition and Impact of Pathologic Complete Response on Prognosis After Neoadjuvant Chemotherapy in Various Intrinsic Breast Cancer Subtypes. J Clin Oncol 30(15):1796–1804. https://doi.org/10.1200/JCO.2011.38.8595
Cortazar P et al (2014) Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet 384(9938):164–172. https://doi.org/10.1016/S0140-6736(13)62422-8
Asselain B et al (2018) Long-term outcomes for neoadjuvant versus adjuvant chemotherapy in early breast cancer: meta-analysis of individual patient data from ten randomised trials. Lancet Oncol 19(1):27–39. https://doi.org/10.1016/S1470-2045(17)30777-5
Maughan KL, Lutterbie MA, Ham PS (2010) Treatment of breast cancer. Natl Libr Med
Moo T-A, Sanford R, Dang C, Morrow M (2018) Overview of Breast Cancer Therapy. PET Clin 13(3):339–354. https://doi.org/10.1016/j.cpet.2018.02.006
Fosu-Mensah N, Peris MS, Weeks HP, Cai J, Westwell AD (2015) Advances in small-molecule drug discovery for triple-negative breast cancer. Future Med Chem 7(15):2019–2039. https://doi.org/10.4155/fmc.15.129
Kalimutho M, Parsons K, Mittal D, López JA, Srihari S, Khanna KK (2015) Targeted Therapies for Triple-Negative Breast Cancer: Combating a Stubborn Disease. Trends Pharmacol Sci 36(12):822–846. https://doi.org/10.1016/j.tips.2015.08.009
Vaz-Luis I et al (2014) Outcomes by Tumor Subtype and Treatment Pattern in Women With Small, Node-Negative Breast Cancer: A Multi-Institutional Study. J Clin Oncol 32(20):2142–2150. https://doi.org/10.1200/JCO.2013.53.1608
Metzger-Filho O et al (2012) Dissecting the Heterogeneity of Triple-Negative Breast Cancer. J Clin Oncol 30(15):1879–1887. https://doi.org/10.1200/JCO.2011.38.2010
Lehmann BD et al (2011) Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest 121(7):2750–2767. https://doi.org/10.1172/JCI45014
Cleator S, Heller W, Coombes RC (2007) Triple-negative breast cancer: therapeutic options. Lancet Oncol. 8(3):235–244. https://doi.org/10.1016/S1470-2045(07)70074-8
Mitra S (2017) MicroRNA Therapeutics in Triple Negative Breast Cancer. Arch Pathol Clin Res 1(1):009–017. https://doi.org/10.29328/journal.hjpcr.1001003
Pareja F, Geyer F.C, Marchiò C, Burke KA, Weigelt B, Reis-Filho JS (2016) Triple-negative breast cancer: the importance of molecular and histologic subtyping, and recognition of low-grade variants. npj Breast Cancer 2(1):16036 https://doi.org/10.1038/npjbcancer.2016.36
Repetto L et al (1996) Tamoxifen and interferon-beta for the treatment of metastatic breast cancer. Breast Cancer Res Treat 39(2):235–238. https://doi.org/10.1007/BF01806190
Kimmick G, Ratain MJ, Berry D, Woolf S, Norton L, Muss HB (2004) Subcutaneously Administered Recombinant Human Interleukin-2 and Interferon Alfa-2a for Advanced Breast Cancer: A Phase II study of the Cancer and Leukemia Group B (CALGB 9041). Invest New Drugs 22(1):83–89. https://doi.org/10.1023/B:DRUG.0000006178.32718.22
Loi S et al (2013) Prognostic and Predictive Value of Tumor-Infiltrating Lymphocytes in a Phase III Randomized Adjuvant Breast Cancer Trial in Node-Positive Breast Cancer Comparing the Addition of Docetaxel to Doxorubicin With Doxorubicin-Based Chemotherapy: BIG 02–98. J Clin Oncol 31(7):860–867. https://doi.org/10.1200/JCO.2011.41.0902
Murakami W et al (2020) Correlation between 18F-FDG uptake on PET/MRI and the level of tumor-infiltrating lymphocytes (TILs) in triple-negative and HER2-positive breast cancer. Eur J Radiol 123:108773. https://doi.org/10.1016/j.ejrad.2019.108773
Rodenhiser DI, Andrews JD, Vandenberg TA, Chambers AF (2011) Gene signatures of breast cancer progression and metastasis. Breast Cancer Res 13(1):201. https://doi.org/10.1186/bcr2791
Loibl S et al (2018) Addition of the PARP inhibitor veliparib plus carboplatin or carboplatin alone to standard neoadjuvant chemotherapy in triple-negative breast cancer (BrighTNess): a randomised, phase 3 trial. Lancet Oncol 19(4):497–509. https://doi.org/10.1016/S1470-2045(18)30111-6
Chaudhary LN (2020) Early stage triple negative breast cancer: Management and future directions. Semin Oncol 47(4):201–208. https://doi.org/10.1053/j.seminoncol.2020.05.006
(2016) Adaptive Randomization of Neratinib in Early Breast Cancer. N Engl J Med 375(16):1591–1594. https://doi.org/10.1056/NEJMc1609993
Rugo HS et al (2016) Adaptive Randomization of Veliparib-Carboplatin Treatment in Breast Cancer. N Engl J Med 375(1):23–34. https://doi.org/10.1056/NEJMoa1513749
Howard FM, Pearson AT, Nanda R (2022) Clinical trials of immunotherapy in triple-negative breast cancer. Breast Cancer Res Treat 195(1):1–15. https://doi.org/10.1007/s10549-022-06665-6
Emens LA et al (2019) Long-term Clinical Outcomes and Biomarker Analyses of Atezolizumab Therapy for Patients With Metastatic Triple-Negative Breast Cancer. JAMA Oncol 5(1):74. https://doi.org/10.1001/jamaoncol.2018.4224
Nanda R et al (2016) Pembrolizumab in Patients With Advanced Triple-Negative Breast Cancer: Phase Ib KEYNOTE-012 Study. J Clin Oncol 34(21):2460–2467. https://doi.org/10.1200/JCO.2015.64.8931
Adams S et al (2019) Pembrolizumab monotherapy for previously treated metastatic triple-negative breast cancer: cohort A of the phase II KEYNOTE-086 study. Ann Oncol 30(3):397–404. https://doi.org/10.1093/annonc/mdy517
Adams S et al (2019) Pembrolizumab monotherapy for previously untreated, PD-L1-positive, metastatic triple-negative breast cancer: cohort B of the phase II KEYNOTE-086 study. Ann Oncol 30(3):405–411. https://doi.org/10.1093/annonc/mdy518
Dirix LY et al (2018) Avelumab, an anti-PD-L1 antibody, in patients with locally advanced or metastatic breast cancer: a phase 1b JAVELIN Solid Tumor study. Breast Cancer Res Treat 167(3):671–686. https://doi.org/10.1007/s10549-017-4537-5
Jovanović B et al (2017) A Randomized Phase II Neoadjuvant Study of Cisplatin, Paclitaxel With or Without Everolimus in Patients with Stage II/III Triple-Negative Breast Cancer (TNBC): Responses and Long-term Outcome Correlated with Increased Frequency of DNA Damage Response Gene Mutations, TNBC Subtype, AR Status, and Ki67. Clin Cancer Res 23(15):4035–4045. https://doi.org/10.1158/1078-0432.CCR-16-3055
Hahnen E et al (2017) Germline Mutation Status, Pathological Complete Response, and Disease-Free Survival in Triple-Negative Breast Cancer. JAMA Oncol 3(10):1378. https://doi.org/10.1001/jamaoncol.2017.1007
Sikov WM et al (2015) Impact of the Addition of Carboplatin and/or Bevacizumab to Neoadjuvant Once-per-Week Paclitaxel Followed by Dose-Dense Doxorubicin and Cyclophosphamide on Pathologic Complete Response Rates in Stage II to III Triple-Negative Breast Cancer: CALGB 40603 (Alliance). J Clin Oncol 33(1):13–21. https://doi.org/10.1200/JCO.2014.57.0572
Tokuda EY, Leal MF, Cardoso Smith MA et al (2020) Understanding and Overcoming Chemoresistance in Triple Negative Breast Cancer: Emerging Role of MicroRNAs. Front Oncol 10:581158. https://doi.org/10.3389/fonc.2020.581158
Corti C, Crimini E, Tarantino P et al (2021) Intratumor Heterogeneity and Its Impact on Patient Stratification in Triple-Negative Breast Cancer. Front Oncol 11:658957. https://doi.org/10.3389/fonc.2021.658957
Liu H, Wang J, Wang J, Wang P (2021) Role of cancer stem cells and signaling pathways in chemoresistance of triple-negative breast cancer. Biomed Pharmacother. 133:110968. https://doi.org/10.1016/j.biopha.2020.110968
Shen L, O’Shea DF, Singh RK et al (2021) Molecular Mechanisms and Clinical Implications of Chemotherapy Drug Resistance in Triple-Negative Breast Cancer. Drug Resist Updat 54:100739. https://doi.org/10.1016/j.drup.2021.100739
Jia Y, Zhang S, Miao L et al (2020) Nanotechnology in Overcoming the Shortcomings of Chemotherapy for Triple-Negative Breast Cancer. Theranostics 10(1):437–456. https://doi.org/10.7150/thno.37018
Bayo J, Castaño MA, Rivera F et al (2020) A comprehensive study of epigenetic alterations in hepatocellular carcinoma identifies potential therapeutic targets. J Hepatol 72(3):523–536. https://doi.org/10.1016/j.jhep.2019.10.03
Wu Y, Liu Q, Li Y et al (2021) Targeted delivery of combined anticancer therapeutics to TNBC cells by nanoparticle-mediated enhanced MRI. Drug Deliv Transl Res 11(3):974–984. https://doi.org/10.1007/s13346-021-00958-6
Samson P, Lewis A, Deodhar K et al (2021) Role of Radiomics in Diagnosis and Management of Breast Cancer: Current Perspectives. Curr Oncol Rep 23(3):26. https://doi.org/10.1007/s11912-021-01004-y
McCann KE, Hurvitz SA (2021) Advances in the Use of PARP Inhibitor Therapy for Breast Cancer. Drugs Context 10. https://doi.org/10.7573/dic.2021-2-6
Rodrigues MA, de Oliveira IS, Marques PZ et al (2020) Triple-negative breast cancer: molecular basis, treatment and resistance mechanisms. J Cancer Res Clin Oncol 146(10):2655–2679. https://doi.org/10.1007/s00432-020-03291-3
Kaklamani VG et al (2015) Phase II neoadjuvant clinical trial of carboplatin and eribulin in women with triple negative early-stage breast cancer (NCT01372579). Breast Cancer Res Treat 151(3):629–638. https://doi.org/10.1007/s10549-015-3435-y
Setyawati MI, Kutty RV, Tay CY, Yuan X, Xie J, Leong DT (2014) Novel Theranostic DNA Nanoscaffolds for the Simultaneous Detection and Killing of Escherichia coli and Staphylococcus aureus. ACS Appl Mater Interfaces 6(24):21822–21831. https://doi.org/10.1021/am502591c
Santos AC et al (2018) Layer-by-Layer coated drug-core nanoparticles as versatile delivery platforms. In: Design and Development of New Nanocarriers. Elsevier. pp. 595–635. https://doi.org/10.1016/B978-0-12-813627-0.00016-8
Zhang X, Liang T, Ma Q (2021) Layer-by-Layer assembled nano-drug delivery systems for cancer treatment. Drug Deliv 28(1):655–669. https://doi.org/10.1080/10717544.2021.1905748
Lu B et al (2019) pH responsive chitosan and hyaluronic acid layer by layer film for drug delivery applications. Prog Org Coatings 135:240–247. https://doi.org/10.1016/j.porgcoat.2019.06.012
Freag MS, Elnaggar YS, Abdelmonsif DA, Abdallah OY (2016) Layer-by-layer-coated lyotropic liquid crystalline nanoparticles for active tumor targeting of rapamycin. Nanomedicine 11(22):2975–2996. https://doi.org/10.2217/nnm-2016-0236
Mu Q et al (2021) Iron oxide nanoparticle targeted chemo-immunotherapy for triple negative breast cancer. Mater Today 50:149–169. https://doi.org/10.1016/j.mattod.2021.08.002
Fahrenholtz CD, Swanner J, Ramirez-Perez M, Singh RN (2017) Heterogeneous Responses of Ovarian Cancer Cells to Silver Nanoparticles as a Single Agent and in Combination with Cisplatin. J Nanomater. 2017:1–11. https://doi.org/10.1155/2017/5107485
Haney MJ et al (2020) Macrophage-Derived Extracellular Vesicles as Drug Delivery Systems for Triple Negative Breast Cancer (TNBC) Therapy. J Neuroimmune Pharmacol 15(3):487–500. https://doi.org/10.1007/s11481-019-09884-9
Byrski T et al (2009) Response to neoadjuvant therapy with cisplatin in BRCA1-positive breast cancer patients. Breast Cancer Res Treat 115(2):359–363. https://doi.org/10.1007/s10549-008-0128-9
Pala R, Anju V, Dyavaiah M, Busi S, Nauli SM (2020) Nanoparticle-Mediated Drug Delivery for the Treatment of Cardiovascular Diseases. Int J Nanomedicine 15:3741–3769. https://doi.org/10.2147/IJN.S250872
Isakoff SJ et al (2015) TBCRC009: A Multicenter Phase II Clinical Trial of Platinum Monotherapy With Biomarker Assessment in Metastatic Triple-Negative Breast Cancer. J Clin Oncol 33(17):1902–1909. https://doi.org/10.1200/JCO.2014.57.6660
Byrski T et al (2014) Pathologic complete response to neoadjuvant cisplatin in BRCA1-positive breast cancer patients. Breast Cancer Res Treat 147(2):401–405. https://doi.org/10.1007/s10549-014-3100-x
Silver DP et al (2010) Efficacy of Neoadjuvant Cisplatin in Triple-Negative Breast Cancer. J Clin Oncol 28(7):1145–1153. https://doi.org/10.1200/JCO.2009.22.4725
Tovey H et al (2015) Managing non-proportionality of hazards (PH) within TNT: a randomised phase III trial of carboplatin compared to docetaxel for patients with metastatic or recurrent locally advanced triple negative (TN) or brca1/2 breast cancer (BC). Trials 16(S2):P150. https://doi.org/10.1186/1745-6215-16-S2-P150
Byrski T et al (2012) Results of a phase II open-label, non-randomized trial of cisplatin chemotherapy in patients with BRCA1-positive metastatic breast cancer. Breast Cancer Res 14(4):R110. https://doi.org/10.1186/bcr3231
Denkert C, Liedtke C, Tutt A, von Minckwitz G (2017) Molecular alterations in triple-negative breast cancer—the road to new treatment strategies. Lancet 389(10087):2430–2442. https://doi.org/10.1016/S0140-6736(16)32454-0
Kennedy RD, Quinn JE, Mullan PB, Johnston PG, Harkin DP (2004) The Role of BRCA1 in the Cellular Response to Chemotherapy. JNCI J Natl Cancer Inst 96(22):1659–1668. https://doi.org/10.1093/jnci/djh312
Yoshida K, Miki Y (2004) Role of BRCA1 and BRCA2 as regulators of DNA repair, transcription, and cell cycle in response to DNA damage. Cancer Sci 95(11):866–871. https://doi.org/10.1111/j.1349-7006.2004.tb02195.x
Jan N et al (2023) Biomimetic cell membrane‐coated poly (lactic‐co‐glycolic acid) nanoparticles for biomedical applications. Bioeng Transl Med 8(2). https://doi.org/10.1002/btm2.10441
Yang C-E, Lee W-Y, Cheng H-W, Chung C-H, Mi F-L, Lin C-W (2019) The antipsychotic chlorpromazine suppresses YAP signaling, stemness properties, and drug resistance in breast cancer cells. Chem Biol Interact 302:28–35. https://doi.org/10.1016/j.cbi.2019.01.033
Hu L et al (2019) The potentiated checkpoint blockade immunotherapy by ROS-responsive nanocarrier-mediated cascade chemo-photodynamic therapy. Biomaterials 223:119469. https://doi.org/10.1016/j.biomaterials.2019.119469
Romero D (2019) Benefit in patients with PD-L1-positive TNBC. Nat Rev Clin Oncol 16(1):6–6. https://doi.org/10.1038/s41571-018-0127-7
Nishino M, Ramaiya NH, Hatabu H, Hodi FS (2017) Monitoring immune-checkpoint blockade: response evaluation and biomarker development. Nat Rev Clin Oncol 14(11):655–668. https://doi.org/10.1038/nrclinonc.2017.88
Kim MH et al (2018) YAP-Induced PD-L1 Expression Drives Immune Evasion in BRAFi-Resistant Melanoma. Cancer Immunol Res 6(3):255–266. https://doi.org/10.1158/2326-6066.CIR-17-0320
Janse van Rensburg HJ et al (2018) The Hippo Pathway Component TAZ Promotes Immune Evasion in Human Cancer through PD-L1. Cancer Res 78(6):1457–1470. https://doi.org/10.1158/0008-5472.CAN-17-3139
Le A et al (2014) Tumorigenicity of hypoxic respiring cancer cells revealed by a hypoxia–cell cycle dual reporter. Proc Natl Acad Sci 111(34):12486–12491. https://doi.org/10.1073/pnas.1402012111
Imran M et al (2021) Fisetin: An anticancer perspective. Food Sci Nutr 9(1):3–16. https://doi.org/10.1002/fsn3.1872
Chen W-J, Tsai J-H, Hsu L-S, Lin C-L, Hong H-M, Pan M-H (2021) Quercetin Blocks the Aggressive Phenotype of Triple Negative Breast Cancer by Inhibiting IGF1/IGF1R-Mediated EMT Program. J Food Drug Anal 29(1):98–112. https://doi.org/10.38212/2224-6614.3090
Umar SM, Patra S, Kashyap A, Arundhathi Dev JR, Kumar L, Prasad CP (2022) Quercetin Impairs HuR-Driven Progression and Migration of Triple Negative Breast Cancer (TNBC) Cells Nutr. Cancer 74(4):1497–1510. https://doi.org/10.1080/01635581.2021.1952628
Yar Saglam AS, Kayhan H, Alp E, Onen HI (2021) Resveratrol enhances the sensitivity of FL118 in triple-negative breast cancer cell lines via suppressing epithelial to mesenchymal transition. Mol Biol Rep 48(1) 475–489 https://doi.org/10.1007/s11033-020-06078-y
Liang Z-J et al (2021) Resveratrol Mediates the Apoptosis of Triple Negative Breast Cancer Cells by Reducing POLD1 Expression. Front Oncol 11. https://doi.org/10.3389/fonc.2021.569295
Cook M, Liang Y, Besch-Williford C, Hyder S (2016) Luteolin inhibits lung metastasis, cell migration, and viability of triple-negative breast cancer cells. Breast Cancer Targets Ther 9:9–19. https://doi.org/10.2147/BCTT.S124860
Cao D et al (2020) Luteolin suppresses epithelial-mesenchymal transition and migration of triple-negative breast cancer cells by inhibiting YAP/TAZ activity. Biomed Pharmacother 129:110462. https://doi.org/10.1016/j.biopha.2020.110462
Greenshields AL et al (2015) Piperine inhibits the growth and motility of triple-negative breast cancer cells. Cancer Lett 357(1):129–140. https://doi.org/10.1016/j.canlet.2014.11.017
Shityakov S et al (2019) Phytochemical and pharmacological attributes of piperine: A bioactive ingredient of black pepper. Eur J Med Chem 176:149–161. https://doi.org/10.1016/j.ejmech.2019.04.002
Guan F, Ding Y, Zhang Y, Zhou Y, Li M, Wang C (2016) Curcumin Suppresses Proliferation and Migration of MDA-MB-231 Breast Cancer Cells through Autophagy-Dependent Akt Degradation. PLoS One 11(1):e0146553. https://doi.org/10.1371/journal.pone.0146553
Mock CD, Jordan BC, Selvam C (2015) Recent advances of curcumin and its analogues in breast cancer prevention and treatment. RSC Adv 5(92):75575–75588. https://doi.org/10.1039/C5RA14925H
Şahin N, Şahin-Bölükbaşı S, Marşan H (2019) Synthesis and antitumor activity of new silver(I)-N-heterocyclic carbene complexes. J Coord Chem 72(22–24):3602–3613. https://doi.org/10.1080/00958972.2019.1697808
Kydd J, Jadia R, Velpurisiva P, Gad A, Paliwal S, Rai P (2017) Targeting Strategies for the Combination Treatment of Cancer Using Drug Delivery Systems. Pharmaceutics 9(4):46. https://doi.org/10.3390/pharmaceutics9040046
Iacopetta D et al (2020) Is the Way to Fight Cancer Paved with Gold? Metal-Based Carbene Complexes with Multiple and Fascinating Biological Features. Pharmaceuticals 13(5):91. https://doi.org/10.3390/ph13050091
Kwon Y-M, Kim SH, Jung Y-S, Kwak J-H (2021) Synthesis and Biological Evaluation of (S)-2-(Substituted arylmethyl)-1-oxo-1,2,3,4-tetrahydropyrazino[1,2-a]indole-3-carboxamide Analogs and Their Synergistic Effect against PTEN-Deficient MDA-MB-468 Cells. Pharmaceuticals 14(10):974. https://doi.org/10.3390/ph14100974
Qin J et al (2022) Design, synthesis and biological evaluation of novel 1,3,4,9-tetrahydropyrano[3,4-b]indoles as potential treatment of triple negative breast cancer by suppressing PI3K/AKT/mTOR pathway. Bioorg Med Chem 55:116594. https://doi.org/10.1016/j.bmc.2021.116594
Hou S et al (2014) Novel Carbazole Inhibits Phospho-STAT3 through Induction of Protein-Tyrosine Phosphatase PTPN6. J Med Chem 57(15):6342–6353. https://doi.org/10.1021/jm4018042
Iacopetta D et al (2017) Multifaceted properties of 1,4-dimethylcarbazoles: Focus on trimethoxybenzamide and trimethoxyphenylurea derivatives as novel human topoisomerase II inhibitors. Eur J Pharm Sci 96:263–272. https://doi.org/10.1016/j.ejps.2016.09.039
Saturnino C et al (2018) Inhibition of Human Topoisomerase II by N, N, N -Trimethylethanammonium Iodide Alkylcarbazole Derivatives. ChemMedChem 13(24):2635–2643. https://doi.org/10.1002/cmdc.201800546
Fedele M, Cerchia L, Chiappetta G (2017) The Epithelial-to-Mesenchymal Transition in Breast Cancer: Focus on Basal-Like Carcinomas. Cancers (Basel) 9(12):134. https://doi.org/10.3390/cancers9100134
Shah V, Bhaliya J, Patel GM (2022) In silico docking and ADME study of deketene curcumin derivatives (DKC) as an aromatase inhibitor or antagonist to the estrogen-alpha positive receptor (Erα+): potent application of breast cancer. Struct Chem 33(2):571–600. https://doi.org/10.1007/s11224-021-01871-2
Jan N et al (2023) Old drug, new tricks: polymer-based nanoscale systems for effective cytarabine delivery. Naunyn Schmiedebergs Arch Pharmacol. https://doi.org/10.1007/s00210-023-02865-z
Ferlay J et al (2015) Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 136(5):E359–E386. https://doi.org/10.1002/ijc.29210
Henriksen EL, Carlsen JF, Vejborg IM, Nielsen MB, Lauridsen CA (2019) The efficacy of using computer-aided detection (CAD) for detection of breast cancer in mammography screening: a systematic review. Acta Radiol 60(1):13–18. https://doi.org/10.1177/0284185118770917
Mansoori B et al (2019) miR-142-3p is a tumor suppressor that inhibits estrogen receptor expression in ER-positive breast cancer. J Cell Physiol 234(9):16043–16053. https://doi.org/10.1002/jcp.28263
Iacopetta D et al (2017) Old Drug Scaffold, New Activity: Thalidomide-Correlated Compounds Exert Different Effects on Breast Cancer Cell Growth and Progression. ChemMedChem 12(5):381–389. https://doi.org/10.1002/cmdc.201600629
Mamnoon B et al (2021) Targeted Polymeric Nanoparticles for Drug Delivery to Hypoxic, Triple-Negative Breast Tumors. ACS Appl Bio Mater 4(2):1450–1460. https://doi.org/10.1021/acsabm.0c01336
Soe ZC et al (2019) Transferrin-Conjugated Polymeric Nanoparticle for Receptor-Mediated Delivery of Doxorubicin in Doxorubicin-Resistant Breast Cancer Cells. Pharmaceutics 11(2):63. https://doi.org/10.3390/pharmaceutics11020063
Yang J et al (2007) Antibody conjugated magnetic PLGA nanoparticles for diagnosis and treatment of breast cancer. J Mater Chem 17(26):2695. https://doi.org/10.1039/b702538f
Jithan A, Madhavi K, Madhavi M, Prabhakar K (2011) Preparation and characterization of albumin nanoparticles encapsulating curcumin intended for the treatment of breast cancer. Int J Pharm Investig 1(2):119. https://doi.org/10.4103/2230-973X.82432
Bhardwaj V, Ankola DD, Gupta SC, Schneider M, Lehr C-M, Kumar MNVR (2009) PLGA Nanoparticles Stabilized with Cationic Surfactant: Safety Studies and Application in Oral Delivery of Paclitaxel to Treat Chemical-Induced Breast Cancer in Rat. Pharm Res 26(11):2495–2503. https://doi.org/10.1007/s11095-009-9965-4
Mukherjee B, Maji R, Dey NS, Satapathy BS, Mondal S (2014) Preparation and characterization of Tamoxifen citrate loaded nanoparticles for breast cancer therapy. Int J Nanomedicine 3107. https://doi.org/10.2147/IJN.S63535.
Zhou Z et al (2017) Sequential delivery of erlotinib and doxorubicin for enhanced triple negative Breast cancer treatment using polymeric nanoparticle. Int J Pharm. 530(1–2):300–307. https://doi.org/10.1016/j.ijpharm.2017.07.085
Zubris KAV, Liu R, Colby A, Schulz MD, Colson YL, Grinstaff MW (2013) In Vitro Activity of Paclitaxel-Loaded Polymeric Expansile Nanoparticles in Breast Cancer Cells. Biomacromolecules 14(6):2074–2082. https://doi.org/10.1021/bm400434h
Dehghan Kelishady P, Saadat E, Ravar F, Akbari H, Dorkoosh F (2015) Pluronic F127 polymeric micelles for co-delivery of paclitaxel and lapatinib against metastatic breast cancer: preparation, optimization and in vitro evaluation. Pharm Dev Technol 20(8):1009–1017. https://doi.org/10.3109/10837450.2014.965323
Zhao Y, Zhang T, Duan S, Davies NM, Forrest ML (2014) CD44-tropic polymeric nanocarrier for breast cancer targeted rapamycin chemotherapy. Nanomedicine Nanotechnology Biol Med 10(6):1221–1230. https://doi.org/10.1016/j.nano.2014.02.015
Bressler EM et al (2018) Biomimetic peptide display from a polymeric nanoparticle surface for targeting and antitumor activity to human triple-negative breast cancer cells. J Biomed Mater Res Part A 106(6):1753–1764. https://doi.org/10.1002/jbm.a.36360
Wu Y et al (2015) Novel Simvastatin-Loaded Nanoparticles Based on Cholic Acid-Core Star-Shaped PLGA for Breast Cancer Treatment. J Biomed Nanotechnol 11(7):1247–1260. https://doi.org/10.1166/jbn.2015.2068
Tseng P-T et al (2018) Peripheral iron levels in children with attention-deficit hyperactivity disorder: a systematic review and meta-analysis. Sci Rep 8(1):788. https://doi.org/10.1038/s41598-017-19096-x
Chen J, Li S, Shen Q, He H, Zhang Y (2011) Enhanced cellular uptake of folic acid–conjugated PLGA–PEG nanoparticles loaded with vincristine sulfate in human breast cancer. Drug Dev Ind Pharm 37(11):1339–1346. https://doi.org/10.3109/03639045.2011.575162
Huo Z et al (2015) Novel nanosystem to enhance the antitumor activity of lapatinib in breast cancer treatment: Therapeutic efficacy evaluation. Cancer Sci 106(10):1429–1437. https://doi.org/10.1111/cas.12737
Das M, Dilnawaz F, Sahoo SK (2011) Targeted nutlin-3a loaded nanoparticles inhibiting p53–MDM2 interaction: novel strategy for breast cancer therapy. Nanomedicine 6(3):489–507. https://doi.org/10.2217/nnm.10.102
Zhou W et al (2014) Aptamer-nanoparticle bioconjugates enhance intracellular delivery of vinorelbine to breast cancer cells. J Drug Target 22(1):57–66. https://doi.org/10.3109/1061186X.2013.839683
Esim O, Hascicek C (2021) Lipid-Coated Nanosized Drug Delivery Systems for an Effective Cancer Therapy. Curr Drug Deliv 18(2):147–161. https://doi.org/10.2174/1567201817666200512104441
Niza et al (2019) Poly(Cyclohexene Phthalate) Nanoparticles for Controlled Dasatinib Delivery in Breast Cancer Therapy. Nanomaterials 9(9):1208. https://doi.org/10.3390/nano9091208
Won Y-W, Patel AN, Bull DA (2014) Cell surface engineering to enhance mesenchymal stem cell migration toward an SDF-1 gradient. Biomaterials 35(21):5627–5635. https://doi.org/10.1016/j.biomaterials.2014.03.070
Wan X et al (2019) Co-delivery of paclitaxel and cisplatin in poly(2-oxazoline) polymeric micelles: Implications for drug loading, release, pharmacokinetics and outcome of ovarian and breast cancer treatments. Biomaterials 192:1–14. https://doi.org/10.1016/j.biomaterials.2018.10.032
Massadeh S et al (2020) Optimized Polyethylene Glycolylated Polymer-Lipid Hybrid Nanoparticles as a Potential Breast Cancer Treatment. Pharmaceutics 12(7):666. https://doi.org/10.3390/pharmaceutics12070666
Katiyar SS, Muntimadugu E, Rafeeqi TA, Domb AJ, Khan W (2016) Co-delivery of rapamycin- and piperine-loaded polymeric nanoparticles for breast cancer treatment. Drug Deliv 23(7):2608–2616. https://doi.org/10.3109/10717544.2015.1039667
Li L et al (2015) Poly(ethylene glycol)-block-poly(ε-caprolactone)- and phospholipid-based stealth nanoparticles with enhanced therapeutic efficacy on murine breast cancer by improved intracellular drug delivery. Int J Nanomedicine 1791. https://doi.org/10.2147/IJN.S75186
Talaei F, Azizi E, Dinarvand R, Atyabi F (2022) Thiolated Chitosan Nanoparticles as a Delivery System for Antisense Therapy: Evaluation against EGFR in T47D Breast Cancer Cells [Retraction]. Int J Nanomedicine 17:3581–3582. https://doi.org/10.2147/IJN.S385585
Aneja et al (2014) Cancer Targeted Magic Bullets for Effective Treatment of Cancer. Recent Patents Anti-Infective Drug Discov 9
Avitabile E, Bedognetti D, Ciofani G, Bianco A, Delogu LG (2018) How can nanotechnology help the fight against breast cancer? Nanoscale 10(25):11719–11731. https://doi.org/10.1039/C8NR02796J
Ferrari M (2005) Cancer nanotechnology: opportunities and challenges. Nat Rev Cancer 5(3):161–171. https://doi.org/10.1038/nrc1566
Gary DJ, Min J, Kim Y, Park K, Won Y-Y (2013) The Effect of N/P Ratio on the In Vitro and In Vivo Interaction Properties of PEGylated Poly[2-(dimethylamino)ethyl methacrylate]-Based siRNA Complexes. Macromol Biosci 13(8):1059–1071. https://doi.org/10.1002/mabi.201300046
Boehnke N et al (2020) Theranostic Layer-by-Layer Nanoparticles for Simultaneous Tumor Detection and Gene Silencing. Angew Chemie 132(7):2798–2805. https://doi.org/10.1002/ange.201911762
Shafiei-Irannejad V et al (2018) Reversion of Multidrug Resistance by Co-Encapsulation of Doxorubicin and Metformin in Poly(lactide-co-glycolide)-d-α-tocopheryl Polyethylene Glycol 1000 Succinate Nanoparticles. Pharm Res 35(6):119. https://doi.org/10.1007/s11095-018-2404-7
Bulbake U, Doppalapudi S, Kommineni N, Khan W (2017) Liposomal Formulations in Clinical Use: An Updated Review. Pharmaceutics 9(4):12. https://doi.org/10.3390/pharmaceutics9020012
Naeem S, Viswanathan G, Bin Misran M (2018) Liposomes as colloidal nanovehicles: on the road to success in intravenous drug delivery. Rev Chem Eng 34(3):365–383 https://doi.org/10.1515/revce-2016-0018
Avvakumova S et al (2019) Does conjugation strategy matter? Cetuximab-conjugated gold nanocages for targeting triple-negative breast cancer cells. Nanoscale Adv 1(9):3626–3638. https://doi.org/10.1039/C9NA00241C
Juneja R, Lyles Z, Vadarevu H, Afonin KA, Vivero-Escoto JL (2021) Multimodal Polysilsesquioxane Nanoparticles for Combinatorial Therapy and Gene Delivery in Triple-Negative Breast Cancer, 1st ed
Riley RS, Day ES (2017) Frizzled7 Antibody-Functionalized Nanoshells Enable Multivalent Binding for Wnt Signaling Inhibition in Triple Negative Breast Cancer Cells. Small 13(26):1700544. https://doi.org/10.1002/smll.201700544
Colombo M et al (2018) Half-Chain Cetuximab Nanoconjugates Allow Multitarget Therapy of Triple Negative Breast Cancer. Bioconjug Chem 29(11):3817–3832. https://doi.org/10.1021/acs.bioconjchem.8b00667
Jamdade VS, Sethi N, Mundhe NA, Kumar P, Lahkar M, Sinha N (2015) Therapeutic targets of triple-negative breast cancer: a review. Br J Pharmacol 172(17):4228–4237. https://doi.org/10.1111/bph.13211
Chen F et al (2013) In Vivo Tumor Targeting and Image-Guided Drug Delivery with Antibody-Conjugated, Radiolabeled Mesoporous Silica Nanoparticles. ACS Nano 7(10):9027–9039. https://doi.org/10.1021/nn403617j
Narayan R, Nayak U, Raichur A, Garg S (2018) Mesoporous Silica Nanoparticles: A Comprehensive Review on Synthesis and Recent Advances. Pharmaceutics 10(3):118. https://doi.org/10.3390/pharmaceutics10030118
Poudel K et al (2019) “Multifaceted NIR-responsive polymer-peptide-enveloped drug-loaded copper sulfide nanoplatform for chemo-phototherapy against highly tumorigenic prostate cancer”, Nanomedicine Nanotechnology. Biol Med 21:102042. https://doi.org/10.1016/j.nano.2019.102042
Park E, Yazdi SJM, Lee J-H (2020) Development of Wearable Temperature Sensor Based on Peltier Thermoelectric Device to Change Human Body Temperature. Sensors Mater 32(9):2959. https://doi.org/10.18494/SAM.2020.2741
Laha D et al (2019) Fabrication of curcumin-loaded folic acid-tagged metal organic framework for triple negative breast cancer therapy in in vitro and in vivo systems. New J Chem 43(1):217–229. https://doi.org/10.1039/C8NJ03350A
Emami F et al (2021) Photoimmunotherapy with cetuximab-conjugated gold nanorods reduces drug resistance in triple negative breast cancer spheroids with enhanced infiltration of tumor-associated macrophages. J Control Release 329:645–664. https://doi.org/10.1016/j.jconrel.2020.10.001
Lin Z, Monteiro-Riviere NA, Riviere JE (2015) Pharmacokinetics of metallic nanoparticles. Wires Nanomed Nanobi 7(2):189–217. https://doi.org/10.1002/wnan.1304
Evans ER, Bugga P, Asthana V, Drezek R (2018) Metallic nanoparticles for cancer immunotherapy. Mater Today 21(6):673–685. https://doi.org/10.1016/j.mattod.2017.11.022
Banstola A, Emami F, Jeong J-H, Yook S (2018) Current Applications of Gold Nanoparticles for Medical Imaging and as Treatment Agents for Managing Pancreatic Cancer. Macromol Res 26(11):955–964. https://doi.org/10.1007/s13233-018-6139-4
Emami F et al (2019) Doxorubicin and Anti-PD-L1 Antibody Conjugated Gold Nanoparticles for Colorectal Cancer Photochemotherapy. Mol Pharm 16(3):1184–1199. https://doi.org/10.1021/acs.molpharmaceut.8b01157
Ahir M et al (2016) Tailored-CuO-nanowire decorated with folic acid mediated coupling of the mitochondrial-ROS generation and miR425-PTEN axis in furnishing potent anti-cancer activity in human triple negative breast carcinoma cells. Biomaterials 76:115–132. https://doi.org/10.1016/j.biomaterials.2015.10.044
Brinkman AM, Wu J, Ersland K, Xu W (2014) Estrogen receptor α and aryl hydrocarbon receptor independent growth inhibitory effects of aminoflavone in breast cancer cells. BMC Cancer 14(1):344. https://doi.org/10.1186/1471-2407-14-344
McLean L et al (2007) Aminoflavone induces oxidative DNA damage and reactive oxidative species-mediated apoptosis in breast cancer cells. Int J Cancer 122(7):1665–1674. https://doi.org/10.1002/ijc.23244
Finlay J, Roberts CM, Lowe G, Loeza J, Rossi JJ, Glackin CA (2015) RNA-Based TWIST1 Inhibition via Dendrimer Complex to Reduce Breast Cancer Cell Metastasis. Biomed Res Int 2015:1–12. https://doi.org/10.1155/2015/382745
Zhang L et al (2018) ZD2-Engineered Gold Nanostar@Metal-Organic Framework Nanoprobes for T 1 -Weighted Magnetic Resonance Imaging and Photothermal Therapy Specifically Toward Triple-Negative Breast Cancer. Adv Healthc Mater 7(24):1801144. https://doi.org/10.1002/adhm.201801144
Kesharwani P, Jain K, Jain NK (2014) Dendrimer as nanocarrier for drug delivery. Prog Polym Sci 39(2):268–307. https://doi.org/10.1016/j.progpolymsci.2013.07.005
Dufes C, Uchegbu I, Schatzlein A (2005) Dendrimers in gene delivery. Adv Drug Deliv Rev 57(15):2177–2202. https://doi.org/10.1016/j.addr.2005.09.017
Kesharwani P et al (2015) PAMAM dendrimers as promising nanocarriers for RNAi therapeutics. Mater Today 18(10):565–572. https://doi.org/10.1016/j.mattod.2015.06.003
Bharti R et al (2017) Somatostatin receptor targeted liposomes with Diacerein inhibit IL-6 for breast cancer therapy. Cancer Lett 388:292–302. https://doi.org/10.1016/j.canlet.2016.12.021
Duwa R, Emami F, Lee S, Jeong J-H, Yook S (2019) Polymeric and lipid-based drug delivery systems for treatment of glioblastoma multiforme. J Ind Eng Chem 79:261–273. https://doi.org/10.1016/j.jiec.2019.06.050
Akbarzadeh A et al (2013) Liposome: classification, preparation, and applications. Nanoscale Res Lett 8(1):102. https://doi.org/10.1186/1556-276X-8-102
Hossen S, Hossain MK, Basher MK, Mia MNH, Rahman MT, Uddin MJ (2019) Smart nanocarrier-based drug delivery systems for cancer therapy and toxicity studies: A review. J Adv Res 15:1–18. https://doi.org/10.1016/j.jare.2018.06.005
Branowska D et al (2018) Synthesis of unsymmetrical disulfanes bearing 1,2,4-triazine scaffold and their in vitro screening towards anti-breast cancer activity. Monatshefte für Chemie – Chem Mon 149(8):1409–1420. https://doi.org/10.1007/s00706-018-2206-y
Mostafa AS, Gomaa RM, Elmorsy MA (2019) Design and synthesis of 2-phenyl benzimidazole derivatives as VEGFR-2 inhibitors with anti-breast cancer activity. Chem Biol Drug Des 93(4):454–463. https://doi.org/10.1111/cbdd.13433
Liu J, Ming B, Gong G-H, Wang D, Bao G-L, Yu L-J (2018) Current research on anti-breast cancer synthetic compounds. RSC Adv 8(8):4386–4416. https://doi.org/10.1039/C7RA12912B
Sideras K et al (2012) North Central Cancer Treatment Group (NCCTG) N0537: Phase II Trial of VEGF-Trap in Patients With Metastatic Breast Cancer Previously Treated With an Anthracycline and/or a Taxane. Clin Breast Cancer 12(6):387–391. https://doi.org/10.1016/j.clbc.2012.09.007
Alvarez RH, Valero V, Hortobagyi GN (2010) Emerging Targeted Therapies for Breast Cancer. J Clin Oncol 28(20):3366–3379. https://doi.org/10.1200/JCO.2009.25.4011
Jia LY, Shanmugam MK, Sethi G, Bishayee A (2016) Potential role of targeted therapies in the treatment of triple-negative breast cancer. Anticancer Drugs 27(3):147–155. https://doi.org/10.1097/CAD.0000000000000328
Levin M (2009) Bioelectric mechanisms in regeneration: Unique aspects and future perspectives. Semin Cell Dev Biol 20(5):543–556. https://doi.org/10.1016/j.semcdb.2009.04.013
McLaughlin KA, Levin M (2018) Bioelectric signaling in regeneration: Mechanisms of ionic controls of growth and form. Dev Biol 433(2):177–189. https://doi.org/10.1016/j.ydbio.2017.08.032
Zhu R, Sun Z, Li C, Ramakrishna S, Chiu K, He L (2019) Electrical stimulation affects neural stem cell fate and function in vitro. Exp Neurol 319:112963. https://doi.org/10.1016/j.expneurol.2019.112963
O’Connor D, Caulfield B (2018) The application of neuromuscular electrical stimulation (NMES) in cancer rehabilitation: current prescription, pitfalls, and future directions. Support Care Cancer 26(11):3661–3663. https://doi.org/10.1007/s00520-018-4269-z
Young CC, Vedadghavami A, Bajpayee AG (2020) Bioelectricity for Drug Delivery: The Promise of Cationic Therapeutics. Bioelectricity 2(2):68–81. https://doi.org/10.1089/bioe.2020.0012
Manabe M, Mie M, Yanagida Y, Aizawa M, Kobatake E (2004) Combined effect of electrical stimulation and cisplatin in HeLa cell death. Biotechnol Bioeng 86(6):661–666. https://doi.org/10.1002/bit.20110
Jossinet J, Schmitt M (1999) A Review of Parameters for the Bioelectrical Characterization of Breast Tissue. Ann NY Acad Sci 873(1):30–41. https://doi.org/10.1111/j.1749-6632.1999.tb09446.x
Sailapu SK et al (2021) Self-activated microbatteries to promote cell death through local electrical stimulation. Nano Energy 83:105852. https://doi.org/10.1016/j.nanoen.2021.105852
Robinson AJ et al (2021) Toward Hijacking Bioelectricity in Cancer to Develop New Bioelectronic Medicine. Adv Ther 4(3):2000248. https://doi.org/10.1002/adtp.202000248
Yoon YN, Lee D-S, Park HJ, Kim J-S (2020) Barium Titanate Nanoparticles Sensitise Treatment-Resistant Breast Cancer Cells to the Antitumor Action of Tumour-Treating Fields. Sci Rep 10(1):2560. https://doi.org/10.1038/s41598-020-59445-x
Hu M, Hong L, He S, Huang G, Cheng Y, Chen Q (2020) Effects of electrical stimulation on cell activity, cell cycle, cell apoptosis and β-catenin pathway in the injured dorsal root ganglion cell. Mol Med Rep. https://doi.org/10.3892/mmr.2020.11058
Love MR, Palee S, Chattipakorn SC, Chattipakorn N (2018) Effects of electrical stimulation on cell proliferation and apoptosis. J Cell Physiol 233(3):1860–1876. https://doi.org/10.1002/jcp.25975
Conta G, Libanori A, Tat T, Chen G, Chen J (2021) Triboelectric Nanogenerators for Therapeutic Electrical Stimulation. Adv Mater 33(26):2007502. https://doi.org/10.1002/adma.202007502
Marino A, Battaglini M, De Pasquale D, Degl’Innocenti A, Ciofani G (2018) Ultrasound-Activated Piezoelectric Nanoparticles Inhibit Proliferation of Breast Cancer Cells. Sci Rep 8(1): 6257 https://doi.org/10.1038/s41598-018-24697-1
Shi X, Chen Y, Zhao Y, Ye M, Zhang S, Gong S (2022) Ultrasound-activable piezoelectric membranes for accelerating wound healing. Biomater Sci 10(3):692–701. https://doi.org/10.1039/D1BM01062J
Yao T et al (2019) The antibacterial effect of potassium-sodium niobate ceramics based on controlling piezoelectric properties. Colloids Surfaces B Biointerfaces 175:463–468. https://doi.org/10.1016/j.colsurfb.2018.12.022
Li C et al (2020) Wireless Electrochemotherapy by Selenium-Doped Piezoelectric Biomaterials to Enhance Cancer Cell Apoptosis. ACS Appl Mater nterfaces 12(31):34505–34513. https://doi.org/10.1021/acsami.0c04666
Genchi GG, Marino A, Rocca A, Mattoli V, Ciofani G (2016) Barium titanate nanoparticles: promising multitasking vectors in nanomedicine. Nanotechnology 27(23):232001. https://doi.org/10.1088/0957-4484/27/23/232001
Shuai C et al (2020) A strawberry-like Ag-decorated barium titanate enhances piezoelectric and antibacterial activities of polymer scaffold. Nano Energy 74:104825. https://doi.org/10.1016/j.nanoen.2020.104825
Min G et al (2021) Ferroelectric-assisted high-performance triboelectric nanogenerators based on electrospun P(VDF-TrFE) composite nanofibers with barium titanate nanofillers. Nano Energy 90:106600. https://doi.org/10.1016/j.nanoen.2021.106600
Wilhelm S et al (2016) Analysis of nanoparticle delivery to tumours. Nat Rev Mater 1(5):16014. https://doi.org/10.1038/natrevmats.2016.14
Champion JA, Mitragotri S (2006) Role of target geometry in phagocytosis. Proc Natl Acad Sci 103(13):4930–4934. https://doi.org/10.1073/pnas.0600997103
Bhar B, Chouhan D, Pai N, Mandal BB (2021) Harnessing Multifaceted Next-Generation Technologies for Improved Skin Wound Healing. ACS Appl Bio Mater 4(11):7738–7763. https://doi.org/10.1021/acsabm.1c00880
Kai H et al (2009) A novel combination of mild electrical stimulation and hyperthermia: General concepts and applications. Int J Hyperth 25(8):655–660. https://doi.org/10.3109/02656730903039605
van Nimwegen MJ, van de Water B (2007) Focal adhesion kinase: A potential target in cancer therapy. Biochem Pharmacol 73(5):597–609. https://doi.org/10.1016/j.bcp.2006.08.011
Huveneers S, Danen EHJ (2009) Adhesion signaling – crosstalk between integrins, Src and Rho. J Cell Sci 122(8):1059–1069. https://doi.org/10.1242/jcs.039446
El Moukhtari SH, Rodríguez-Nogales C, Blanco-Prieto MJ (2021) Oral lipid nanomedicines: Current status and future perspectives in cancer treatment. Adv Drug Deliv Rev 173:238–251. https://doi.org/10.1016/j.addr.2021.03.004
Yan L, Shen J, Wang J, Yang X, Dong S, Lu S (2020) Nanoparticle-Based Drug Delivery System: A Patient-Friendly Chemotherapy for Oncology. Dose-Response 18(3):155932582093616. https://doi.org/10.1177/1559325820936161
Miller AD (2013) Lipid-Based Nanoparticles in Cancer Diagnosis and Therapy. J Drug Deliv 2013:1–9. https://doi.org/10.1155/2013/165981
Aqil F, Munagala R, Agrawal AK, Gupta R (2019) Anticancer Phytocompounds. In: New Look to Phytomedicine. Elsevier, pp. 237–272. https://doi.org/10.1016/B978-0-12-814619-4.00010-0.
Talluri SV, Kuppusamy G, Karri VVSR, Tummala S, Madhunapantula SV (2016) Lipid-based nanocarriers for breast cancer treatment – comprehensive review. Drug Deliv 23(4):1291–1305. https://doi.org/10.3109/10717544.2015.1092183
Čerpnjak K, Zvonar A, Gašperlin M, Vrečer F (2013) Lipid-based systems as a promising approach for enhancing the bioavailability of poorly water-soluble drugs. Acta Pharm 63(4):427–445. https://doi.org/10.2478/acph-2013-0040
Kushwah V, Katiyar SS, Agrawal AK, Gupta RC, Jain S (2018) Co-delivery of docetaxel and gemcitabine using PEGylated self-assembled stealth nanoparticles for improved breast cancer therapy. Nanomedicine Nanotechnology Biol Med 14(5):1629–1641. https://doi.org/10.1016/j.nano.2018.04.009
Kushwah V et al (2018) Implication of linker length on cell cytotoxicity, pharmacokinetic and toxicity profile of gemcitabine-docetaxel combinatorial dual drug conjugate. Int J Pharm 548(1):357–374. https://doi.org/10.1016/j.ijpharm.2018.07.016
Lim SB, Banerjee A, Önyüksel H (2012) Improvement of drug safety by the use of lipid-based nanocarriers. J Control Release 163(1):34–45. https://doi.org/10.1016/j.jconrel.2012.06.002
Darwis Y, Ali Khan A, Mudassir J, Mohtar N (2013) Advanced drug delivery to the lymphatic system: lipid-based nanoformulations. Int J Nanomedicine 2733. https://doi.org/10.2147/IJN.S41521
Kumar DN et al (2022) Exosomes as Emerging Drug Delivery and Diagnostic Modality for Breast Cancer: Recent Advances in Isolation and Application. Cancers (Basel) 14(6):1435. https://doi.org/10.3390/cancers14061435
Antimisiaris S, Mourtas S, Marazioti A (2018) Exosomes and Exosome-Inspired Vesicles for Targeted Drug Delivery. Pharmaceutics 10(4):218. https://doi.org/10.3390/pharmaceutics10040218
Chen Y-S, Lin E-Y, Chiou T-W, Harn H-J (2020) Exosomes in clinical trial and their production in compliance with good manufacturing practice. Tzu Chi Med J 32(2):113. https://doi.org/10.4103/tcmj.tcmj_182_19
Gupta A, Eral HB, Hatton TA, Doyle PS (2016) Nanoemulsions: formation, properties and applications. Soft Matter 12(11):2826–2841. https://doi.org/10.1039/C5SM02958A
Kaur T, Slavcev R (2013) Solid Lipid Nanoparticles: Tuneable Anti-Cancer Gene/Drug Delivery Systems. In: Novel Gene Therapy Approaches. InTech. https://doi.org/10.5772/54781
Gabizon AA, Shmeeda H, Zalipsky S (2006) Pros and Cons of the Liposome Platform in Cancer Drug Targeting. J Liposome Res 16(3):175–183. https://doi.org/10.1080/08982100600848769
Iqbal MA, Md S, Sahni JK, Baboota S, Dang S, Ali J (2012) Nanostructured lipid carriers system: Recent advances in drug delivery. J Drug Target 20(10):813–830. https://doi.org/10.3109/1061186X.2012.716845
Persano F, Gigli G, Leporatti S (2021) Lipid-polymer hybrid nanoparticles in cancer therapy: current overview and future directions. Nano Express 2(1):012006. https://doi.org/10.1088/2632-959X/abeb4b
Wang C et al (2018) Triple negative breast cancer in Asia: An insider’s view. Cancer Treat Rev 62:29–38. https://doi.org/10.1016/j.ctrv.2017.10.014
Kim B, Pena CD, Auguste DT (2019) Targeted Lipid Nanoemulsions Encapsulating Epigenetic Drugs Exhibit Selective Cytotoxicity on CDH1 – /FOXM1 + Triple Negative Breast Cancer Cells. Mol Pharm 16(5):1813–1826. https://doi.org/10.1021/acs.molpharmaceut.8b01065
Xu H et al (2020) Nano-puerarin regulates tumor microenvironment and facilitates chemo- and immunotherapy in murine triple negative breast cancer model. Biomaterials 235:119769. https://doi.org/10.1016/j.biomaterials.2020.119769
Han B et al (2021) Elemene Nanoemulsion Inhibits Metastasis of Breast Cancer by ROS Scavenging. Int J Nanomedicine 16:6035–6048. https://doi.org/10.2147/IJN.S327094
Saraiva SM et al (2021) Edelfosine nanoemulsions inhibit tumor growth of triple negative breast cancer in zebrafish xenograft model. Sci Rep 11(1):9873. https://doi.org/10.1038/s41598-021-87968-4
Guo P et al (2019) Dual complementary liposomes inhibit triple-negative breast tumor progression and metastasis. Sci Adv 5(3), https://doi.org/10.1126/sciadv.aav5010
Chaudhuri A et al (2022) Lipid-Based Nanoparticles as a Pivotal Delivery Approach in Triple Negative Breast Cancer (TNBC) Therapy. Int J Mol Sci 23(17):10068. https://doi.org/10.3390/ijms231710068
Chen M et al (2021) Detachable Liposomes Combined Immunochemotherapy for Enhanced Triple-Negative Breast Cancer Treatment through Reprogramming of Tumor-Associated Macrophages. Nano Lett 21(14):6031–6041. https://doi.org/10.1021/acs.nanolett.1c01210
Alawak M et al (2021) ADAM 8 as a novel target for doxorubicin delivery to TNBC cells using magnetic thermosensitive liposomes. Eur J Pharm Biopharm 158:390–400. https://doi.org/10.1016/j.ejpb.2020.12.012
El-Senduny FF et al (2021) Azadiradione-loaded liposomes with improved bioavailability and anticancer efficacy against triple negative breast cancer. J Drug Deliv Sci Technol 65:102665. https://doi.org/10.1016/j.jddst.2021.102665
Guney Eskiler G, Cecener G, Egeli U, Tunca B (2018) Synthetically Lethal BMN 673 (Talazoparib) Loaded Solid Lipid Nanoparticles for BRCA1 Mutant Triple Negative Breast Cancer. Pharm Res 35(11):218. https://doi.org/10.1007/s11095-018-2502-6
Siddhartha VT, Pindiprolu SKSS, Chintamaneni PK, Tummala S, Nandha Kumar S (2018) RAGE receptor targeted bioconjuguate lipid nanoparticles of diallyl disulfide for improved apoptotic activity in triple negative breast cancer: in vitro studies. Artif Cells Nanomedicine Biotechnol 46(2):387–397 https://doi.org/10.1080/21691401.2017.1313267.
Kothari IR, Mazumdar S, Sharma S, Italiya K, Mittal A, Chitkara D (2019) Docetaxel and alpha-lipoic acid co-loaded nanoparticles for cancer therapy. Ther Deliv 10(4):227–240. https://doi.org/10.4155/tde-2018-0074
Pindiprolu SKSS, Chintamaneni PK, Krishnamurthy PT, Ratna Sree Ganapathineedi K (2019) Formulation-optimization of solid lipid nanocarrier system of STAT3 inhibitor to improve its activity in triple negative breast cancer cells. Drug Dev Ind Pharm 45(2):304–313 https://doi.org/10.1080/03639045.2018.1539496
Pindiprolu SKSS, Krishnamurthy PT, Ghanta VR, Chintamaneni PK (2020) Phenyl boronic acid-modified lipid nanocarriers of niclosamide for targeting triple-negative breast cancer. Nanomedicine 15(16):1551–1565 https://doi.org/10.2217/nnm-2020-0003
Zhang T et al (2017) Dual-targeted hybrid nanoparticles of synergistic drugs for treating lung metastases of triple negative breast cancer in mice. Acta Pharmacol Sin 38(6):835–847. https://doi.org/10.1038/aps.2016.166
Zhou Z, Kennell C, Lee J-Y, Leung Y-K, Tarapore P (2017) Calcium phosphate-polymer hybrid nanoparticles for enhanced triple negative breast cancer treatment via co-delivery of paclitaxel and miR-221/222 inhibitors. Nanomedicine Nanotechnology Biol Med 13(2):403–410. https://doi.org/10.1016/j.nano.2016.07.016
Garg NK et al (2017) Functionalized Lipid-Polymer Hybrid Nanoparticles Mediated Codelivery of Methotrexate and Aceclofenac: A Synergistic Effect in Breast Cancer with Improved Pharmacokinetics Attributes. Mol Pharm 14(6):1883–1897. https://doi.org/10.1021/acs.molpharmaceut.6b01148
Naseri Z, Kazemi Oskuee R, Jaafari MR, Forouzandeh M (2018) Exosome-mediated delivery of functionally active miRNA-142-3p inhibitor reduces tumorigenicity of breast cancer in vitro and in vivo. Int J Nanomedicine 13:7727–7747 https://doi.org/10.2147/IJN.S182384
Gong C et al (2019) Functional exosome-mediated co-delivery of doxorubicin and hydrophobically modified microRNA 159 for triple-negative breast cancer therapy. J Nanobiotechnology 17(1):93. https://doi.org/10.1186/s12951-019-0526-7
Yu M et al (2019) Targeted exosome-encapsulated erastin induced ferroptosis in triple negative breast cancer cells. Cancer Sci 110(10):3173–3182. https://doi.org/10.1111/cas.14181
Pedro IDR et al (2019) Optimization and in vitro/in vivo performance of paclitaxel-loaded nanostructured lipid carriers for breast cancer treatment. J Drug Deliv Sci Technol 54:101370. https://doi.org/10.1016/j.jddst.2019.101370
Zhang Q et al (2019) Construction and in vitro and in vivo evaluation of folic acid-modified nanostructured lipid carriers loaded with paclitaxel and chlorin e6. Int J Pharm 569:118595. https://doi.org/10.1016/j.ijpharm.2019.118595
Lages EB et al (2020) Co-delivery of doxorubicin, docosahexaenoic acid, and α-tocopherol succinate by nanostructured lipid carriers has a synergistic effect to enhance antitumor activity and reduce toxicity. Biomed Pharmacother 132:110876. https://doi.org/10.1016/j.biopha.2020.110876
Gadag S et al (2021) Development and preclinical evaluation of microneedle-assisted resveratrol loaded nanostructured lipid carriers for localized delivery to breast cancer therapy. Int J Pharm 606:120877. https://doi.org/10.1016/j.ijpharm.2021.120877
Rwei AY, Lee J-J, Zhan C et al (2020) Repeatable and adjustable on-demand sciatic nerve block with phototriggerable liposomes. Proc Natl Acad Sci USA 117(49):31197–31206. https://doi.org/10.1073/pnas.2011843117
Zhang X, Dai Z, Jin Z et al (2020) Enhancing Triple-Negative Breast Cancer Immunotherapy by ICP-MS-Instructed pH-Responsive PD-L1-Targeted Liposome. Nano Lett 20(11):8013–8023. https://doi.org/10.1021/acs.nanolett.0c03516
Zuo T, Gong Y, Chen Z et al (2021) A Small-Sized Tumor Microenvironment-Responsive Nanodrug for Deep Penetration and Antimetastasis Therapy. Small 17(8):2006813. https://doi.org/10.1002/smll.202006813
Cheng L, He W, Gong H et al (2020) PEGylated Micelle Nanoparticles Encapsulating ICG for Photothermal Therapy in Colorectal Cancer. Bioconjug Chem 31(6):1924–1932. https://doi.org/10.1021/acs.bioconjchem.0c00235
El-Sayed A, Khalafallah S, Adel MM, Elkhodiry A (2020) Enhancement of the cytotoxicity of anti-cancer drugs using stable nano-sized lipid carriers. Drug Deliv Transl Res 10(3):718–732. https://doi.org/10.1007/s13346-020-00724-w
Gao S, Tang G, Hua D et al (2020) Systemic immune-inflammation index (SII) and platelet-to-lymphocyte ratio (PLR) are associated with prognosis of epithelial ovarian cancer. Gynecol Oncol 157(1):215–220. https://doi.org/10.1016/j.ygyno.2020.02.012
Liu X, Wang X, Zheng Y et al (2021) Nanocarriers for Ferroptosis Therapy: A Strategy for Precision Medicine in Cancer Treatment. Biomater Sci 9(7):2347–2364. https://doi.org/10.1039/d0bm01722a
Zheng S, Gong Z, Cheng Q et al (2020) A novel alginate-hydrogel encapsulated nano-liposome platform for local administration of anticancer drugs to brain tumors. Drug Deliv Transl Res 10(6):1673–1681. https://doi.org/10.1007/s13346-020-00760-6
Huang C, Zhang H, Bai L et al (2021) Exploring Mechanisms of ASO Delivery and Distribution in Solid Tumors for Optimized ASO Therapeutics. Adv Sci (Weinh) 8(6):2002985. https://doi.org/10.1002/advs.202002985
Kang JH, Toita R, Murata M et al (2020) Extracellular vesicle mimetics technology for extracellular vesicle purification, exosome engineering, and exosome-mimetics drug delivery. Biotechnol Bioeng 117(12):3873–3887. https://doi.org/10.1002/bit.27542
Acknowledgments
Not applicable.
Funding
This work received no associated funding.
Author information
Authors and Affiliations
Contributions
SM contributed to the conception and design of this review and the completion of the figures and tables. VS contributed to the making cover figure and doing work on plagiarism. GP and NJ and CA contributed to the reading of the article and final approval of the submitted review. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
The authors declare that no funds, grants or other support were received during the preparation of this manuscript.
Ethical Statement
Not applicable for both human and animal studies.
Conflict of interest
The authors of this review affirm that they carried out the study without any affiliations or financial associations that could be perceived as possible conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mehta, S., Shah, V., Patel, G. et al. A holistic review of recent advances in nano-based drug delivery systems for the treatment of triple-negative breast cancer (TNBC). J Nanopart Res 26, 87 (2024). https://doi.org/10.1007/s11051-024-06000-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-024-06000-8