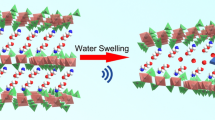
Abstract
In order to explore the influence of freeze-drying process parameters on the size of zirconium phosphate (α-ZrP) nanoparticles during freeze-drying, different process parameters were selected and orthogonal experiments were designed to prepare nano α-ZrP with different particle sizes. The structures of nano α-ZrP are characterized by the field emission scanning electron microscope (SEM), and the tribological properties of nano α-ZrP are studied on a tribometer. The orthogonal experimental results indicated that the order of process parameters affecting the size of nano α-ZrP is freezing method > material tray area > reaction temperature. The freezing method has the greatest influence on the size of nano α-ZrP. The particle size is around 20 nm under vacuum freezing, 40 nm under liquid nitrogen freezing, and 60 nm under refrigerator freezing. The reaction temperature of 98 °C, the material disk area of 80 mm2, and vacuum freezing were found to be the optimal process parameters for the preparation of small size nano α-ZrP by freeze-drying. Different amounts of granular nano α-ZrP were added to the base oil; when the addition amount was 2%, the friction coefficient was the smallest, which could be reduced by 34.6%. Friction experiments were conducted on three distinct morphologies of nano α-ZrP (granular, lamellar, and rod); the results show that the friction performance of nano α-ZrP with granular morphology is better than that of the other two crystal morphologies.
Graphical abstract







Similar content being viewed by others
References
Debkumar C, Swaathi S, Kakarla RR (2019) Integration of biological pre-treatment methods for increased resource replace resource with energy recovery from paper and pulp biosludge. J Microbiol Methods 160:93–10. https://doi.org/10.1016/j.mimet.2019.03.015R
Yi S, Ge XY, Li JJ (2020) Development and prospects of liquid superlubricity. Tsinghua Sci Technol 60(08):617–629. https://doi.org/10.16511/j.cnki.qhdxxb.2020.25.023
Holmberg K, Andersson P, Erdemir A (2012) Global energy consumption due to friction in passenger cars. Tribol Int 47:221–234. https://doi.org/10.1016/j.triboint.2011.11.022
Padgurskas J, Rukuiza R (2013) Tribological properties of lubricant additives of Fe, Cu and Co nanoparticles. Tribol Int 60(3):224–232. https://doi.org/10.1016/j.triboint.2012.10.024
Kuang X, Yin B, Yang X (2023) Preparation and tribological behavior of TiO2@ WO3: Eu3+ powders as lubricant nano-additive for steel-steel contacts. J Nanopart Res 25:3. https://doi.org/10.1007/s11051-022-05646-6
Wu H, Johnson B, Wang L (2017) High-efficiency preparation of oil-dispersible MoS2 nanosheets with superior anti-wear property in ultralow concentration. J Nanopart Res 19:339. https://doi.org/10.1007/s11051-017-4035-z
Kuang X, Yin B, Yang XP (2022) Study of dispersion stability and wear property of lubricating oil blends added with nanographene for a diesel engine. Lubr Sci 34(5):291–302. https://doi.org/10.1002/ls.1589
Kai F (2021) Preparation of zirconium hydrogen phosphate coatings on sandblasted/ acid-etched titanium for enhancing its osteoinductivity and friction/corrosion resistance. Int J Nanomed 16. https://doi.org/10.2147/ijn.s337028
Kakarla R, Hemavathi B, Geetha R (2019) Organic conjugated polymer-based functional nanohybrids: synthesis methods, mechanisms and its applications in electrochemical energy storage supercapacitors and solar cells. Polym Compos Func Naonopar 11:357–379. https://doi.org/10.1016/B978-0-12-814064-2.00011-1
He XL, Xiao XP (2014) α-Zirconium phosphate nanoplatelets as lubricant additives. Colloids Surf A Physicochem Eng Aspects 452:32–38. https://doi.org/10.1016/j.colsurfa.2014.03.041
Gulzar M, Masjuki H, Kalam A (2016) Tribological performance of nanoparticles as lubricating oil additives. J Nanopart Res 18:223. https://doi.org/10.1007/s11051-016-3537-4
Kolodziejczyk L, Martínez-Martínez D, Rojas T (2007) Surface-modified Pd nanoparticles as a superior additive for lubrication. J Nanopart Res 9:639–645. https://doi.org/10.1007/s11051-006-9124-3
Gao C, Wang Y, Hu D et al (2013) Tribological properties of magnetite nanoparticles with various morphologies as lubricating additives. J Nanopart Res 15:1502. https://doi.org/10.1007/s11051-013-1502-z
Bakunin V, Suslov A, Kuzmina G et al (2004) Synthesis and application of inorganic nanoparticles as lubricant components a review. J Nanopart Res 6:273–284. https://doi.org/10.1023/B:NANO.0000034720.79452.e3
Wan Q, Jin Y, Sun P et al (2014) Rheological and tribological behaviour of lubricating oils containing platelet MoS2 nanoparticles. J Nanopart Res 16:2386. https://doi.org/10.1007/s11051-014-2386-2
Li W, Zheng S, Cao B et al (2011) Friction and wear properties of ZrO2/SiO2 composite nanoparticles. J Nanopart Res 13:2129–2137. https://doi.org/10.1007/s11051-010-9970-x
Chen Y, Wang X, Abraham C (2020) Nanoparticle α-ZrP enhanced superhydrophobicity. Solvent Extr Ion Exch 38(6):645–655. https://doi.org/10.1080/07366299.2020.1780702
Zhu X, Yan Q, Yu Y (2021) Orientation of ultrathin α-ZrP nanosheets in aqueous epoxy resin for anticorrosive coatings. ACS Appl Nano Mater 4:5413–5424. https://doi.org/10.1021/acsanm.1c00741
Jiang F, Sun H (2020) Dispersion-tribological property relationship in mineral oils containing 2D layered α-zirconium phosphate nanoplatelets. Friction 8:695–707. https://doi.org/10.1007/s40544-019-0294-2
Zhang XS, Xu H (2013) Hydrothermal synthesis of copper zirconium phosphate hydrate [Cu(OH)2Zr(HPO4)2·2H2O] and an investigation of its lubrication properties in grease. ACS Appl Mater Interfaces 5(16):7989–7994. https://doi.org/10.1021/am402073b
Sun LY, Boo WJ, Sue HJ, Clearfield A (2007) Preparation of α-zirconium phosphate nanoplatelets with wide variations in aspect ratios. New J Chem 31(1):39–43. https://doi.org/10.1039/b604054c
Ma H, Zhang X, Xu H (2015) Tribological properties of layered A-zirconium phosphate as lithium grease additive. Chem Asian J 27(3):1133–1137. https://doi.org/10.14233/ajchem.2015.18474
Clearfield A, Smith GD (1969) Crystallography and structure of α-zirconium bis (monohydrogen orthophosphate) mono-hydrate. Chem Asian J 8(3):431–436
Zhou G, Wang Q, Zeng R (2014) Preparation and application of zirconium phosphate and its derivatives. Prog Chem 26(01):87–99. https://doi.org/10.7536/PC130638
Clearfield A, Blessing RH, Stynes JA (1968) New crystalline phases of zirconium phosphate possessing ion-exchange properties. J Inorg Nucl Chem 30(8):2249–2258
Kaňa A, Loula M, Mestek O (2021) Controlled preparation of arsenic nanoparticles. J Nanopart Res 23:239. https://doi.org/10.1007/s11051-021-05356-5
Cao Z, Sun L (2011) Synthesis and characterization of the layered zirconium phosphate and its intercalation study. Adv Mat Res 233–235:1809–1813. https://doi.org/10.4028/www.scientific.net/AMR.233-235.1809
Peng R, Han S, Zeng Q (2016) Synthesis and characterization of vacuum freeze-dried copper nano-powder. J Vac Sci Technol 36(2):217–222. https://doi.org/10.13922/j.cnki.cjovst.2016.02.16
Yi S, Peng R (2018) Preparation of nano-MoS2 powders by vacuum freeze-drying. Vacuum 55(6):80–83. https://doi.org/10.13385/j.cnki.vacuum.2018.06.18
Mazan MO, Marrero-Jerez J, Soldati A (2015) Fe-doped ceria nano-powders synthesized by freeze-drying precursor method for electrocatalytic applications. Int J Hydrog Energy 40(10):3981–3989. https://doi.org/10.1016/j.ijhydene.2015.01.006
Peng R, Guo J, Han S (2020) Tribological performance of freeze-drying nano-copper particle as additive of paroline oil. Mater Res Express 7(2):025028. https://doi.org/10.1088/2053-1591/ab726c
Demina EN, Safronova OV, Kuprina IK (2021) Research of the mineral composition of freeze-dried plant powders. IOP Conference Series: Environ Earth Sci 848(1):012040(5pp). https://doi.org/10.1088/1755-1315/848/1/012040
Hu W, Dong Z, Wang H (2021) Microstructure refinement and mechanical properties improvement in the W-Y2O3 alloys via optimized freeze-drying. Int J Refract Met H 95:105453. https://doi.org/10.1016/j.ijrmhm.2020.105453
Zhecheva E (2010) Particle size distribution and electrochemical properties of LiFePO4 prepared by a freeze-drying method. J Phys Chem Solids 71(5):848–853. https://doi.org/10.1016/j.jpcs.2010.02.012
Xu C, Zhang S, Peng R (2008) Today and tomorrow of vacuum freeze-drying technology. Vacuum (2):1–11. https://doi.org/10.13385/j.cnki.vacuum.2008.02.016
Bovone G, Kawale S, Bernini C (2016) Freeze drying technique to prepare doped nanosized B powder. Dry Technol 34(8):923–929. https://doi.org/10.1080/07373937.2015.1086783
Wang H, Tian T, Yuan L (2013) Synthesis of Nd: YAG nano-powder by sol-gel plus freeze-drying method and fabrication of transparent ceramic. Bull Chinese Ceramic Soc 32(12):2564–2567. https://doi.org/10.16552/j.cnki.issn1001-1625.2013.12.023
Zhou Z, Yang F, Chen J (2011) Synthesis of yttrium aluminum garnet by co-precipitation freeze drying. Laser Optoelectron Prog 48(10):136–139. https://doi.org/10.3788/LOP48.101602
Mann R, Laishram K (2015) Sol freeze dry Nd: YAG nanopowder synthesis and sinterability studies. Defence Sci J 65(5). https://doi.org/10.14429/dsj.65.8960
Gazi H, Jie L, Han G (2015) Preparation of nano-sized copper β-resorcylate (β-Cu) and its excellent catalytic activity for the thermal decomposition of ammonium perchlorate. Propell Explos Pyrot 40(6):848–853. https://doi.org/10.1002/prep.201500006
Dai Y, Niu W, Zhang X (2018) Tribological properties of layered zirconium phosphate as an additive in anhydrous calcium grease. Acta Petrol Sin 34(4):776–782. https://doi.org/10.3969/j.issn.1001-8719.2018.04.018
Wu Z, Hou K, Wang J (2020) α-ZrP preparation and tribological properties of surfactant modified α-ZrP layered nanosheets. Tribology 195(01):92–99. https://doi.org/10.16078/j.tribology.2019088
Funding
This work was financially supported by National Natural Science Foundation of China (52175113) and Shaanxi Province science and technology planning project (2022GY-214).
Author information
Authors and Affiliations
Contributions
RP: writing—review and editing, writing—original draft. JL: writing—original draft. ZY: software. WW: software. HZ: validation. JG: methodology. WC: writing—review and editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
1. Freeze-drying method can make smaller and more uniform nano α-ZrP particles.
2. Process parameters of the freeze drying affect the size of nano α-ZrP particles.
3. The friction coefficient of globular nano α-ZrP oil sample can be reduced to 0.085.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Runling, P., Jinyue, L., Zhuoyu, Y. et al. Preparation of nano-zirconium phosphate by freeze-drying method and its tribological properties. J Nanopart Res 26, 9 (2024). https://doi.org/10.1007/s11051-023-05915-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-023-05915-y