Abstract
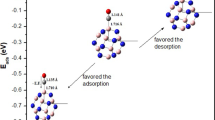
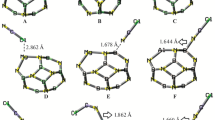
This work studied the adsorption of nitrogen monoxide gas on Cu-modified B12N12 nanocage as a chemical sensor application. We performed quantum chemical calculations using density functional theory (DFT) at the B3LYP-D3/6-31G(d,p) level. Seven adsorption configurations of NO molecule on pristine B12N12 were tested to find the best interaction mode. The modification of B12N12 with copper resulted in five optimized structures: CuB11N12 and B12N11Cu (doped), Cu@b66 and Cu@b64 (decorated), and Cu@B12N12 (encapsulated). The quantum molecular dynamic analysis revealed that almost all isolated systems are stable. The results indicate that the NO gas weakly physisorbed on pure B12N12 nanocage, but the Cu-modifications improved adsorption performance. We also found that, over B12N12, the NO molecule prefers to adsorb on the O site rather than the N site. In addition, our findings showed that Cu@B12N12 has high electronic sensitivity (ΔEgap = 87.4%) and great conductometric potential (∆Φ = 48.9%) towards NO gas, when compared to the other modified systems. Regarding selective detection of NO gas among other gas molecules (CH4, N2O, SO2, CO2, H2, and NH3), the Cu@B12N12 system showed high selectivity for NO detection. Finally, the results for energy gap and work function demonstrated the capability of Cu@B12N12 as a conductometric and work function sensor material type for applications in the selective detection of NO gas.
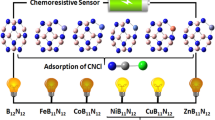
Graphical abstract
Schematic representation of the detection of toxic gases onto Cu@B12N12 surface.









Similar content being viewed by others
Data Availability
The authors confirm that the data supporting the findings of this study are available within the article.
References
Kroto HW, Heath JR, O’Brien SC, Curl RF, Smalley RE (1985) C60: Buckminsterfullerene. Nature 318:162–163. https://doi.org/10.1038/318162a0
Ijima S (1991) Helical microtubules of graphitic carbon. Nature 354:56–58. https://doi.org/10.1038/354056a0
Qi P, Vermesh O, Grecu M, Javey A, Wang Q, Dai H, Peng S, Cho KJ (2003) Toward large arrays of multiplex functionalized carbon nanotube sensors for highly sensitive and selective molecular detection. Nano Lett 3:347–351. https://doi.org/10.1021/nl034010k
Hu C, Hu S (2009) Carbon nanotube-based electrochemical sensors: principles and applications in biomedical systems. J Sens 2009:1–40. https://doi.org/10.1155/2009/187615
Shettia NP, Mishra A, Basu S, Aminabhavi TM (2021) Versatile fullerenes as sensor materials. Mater Today Chem 20:100454. https://doi.org/10.1016/j.mtchem.2021.100454
Chuang C-W, Shih J-S (2001) Preparation and application of immobilized C60-glucose oxidase enzyme in fullerene C60-coated piezoelectric quartz crystal glucose sensor. Sensors Actuators B 81:1–8. https://doi.org/10.1016/S0925-4005(01)00914-5
Keshtkar S, Rashidi A, Kooti M, Askarieh M, Pourhashem S, Ghasemy E, Izadi N (2018) A novel highly sensitive and selective H2S gas sensor at low temperatures based on SnO2 quantum dots-C60 nanohybrid: experimental and theory study. Talanta 188:531–539. https://doi.org/10.1016/j.talanta.2018.05.099
Paine RT, Narula CK (1990) Synthetic routes to boron nitride. Chem Rev 90:73–91. https://doi.org/10.1021/cr00099a004
Oku T, Hirano T, Kuno M, Kusunose T, Niihare K, Suganuma K (2000) Synthesis, atomic structures and properties of carbon and boron nitride fullerene materials. Mater Sci Eng B 74:206–217. https://doi.org/10.1016/S0921-5107(99)00563-2
Seifert G, Fowler RW, Mitchell D, Porezag D, Frauenheim Th (1997) Boron-nitrogen analogues of the fullerenes: electronic and structural properties. Chem Phys Lett 268:352–358. https://doi.org/10.1016/S0009-2614(97)00214-5
Wu H, Xu X, Jiao H, Zhang F, Jia J (1997) Structure and stability of boron nitride cages. Chin Sci Bull 48:1102–1107
Strout DL (2000) Structure and stability of boron nitrides: isomers of B12N12. J Phys Chem A 104:3364–3366. https://doi.org/10.1021/jp994129a
Xie W, Wang J, Wang J, Wu X, Wang Z, Zhang R-Q (2020) High-Angular-momentum orbitals and superatomic characteristics of boron-nitrogen cages. J Phys Chem C 124:3881–3885. https://doi.org/10.1021/acs.jpcc.9b11351
Strout DL (2001) Structure and stability of boron nitrides: the crossover between rings and cages. J Phys Chem A 104:3364–3366. https://doi.org/10.1021/jp003187p
Jensen F, Toftlund H (1993) Structure and stability of C24 and B12N12 isomers. Chem Phys Lett 201:89–96. https://doi.org/10.1016/0009-2614(93)85039-Q
Golberg D, Bando Y, Stephan O, Kurashima K (1998) Octahedral boron nitride fullerenes formed by electron beam irradiation. Appl Phys Lett 73:2441–2443. https://doi.org/10.1063/1.122475
Oku T, Nishiwaki A, Narita I (2004) Formation and atomic structure of B12N12 nanocage clusters studied by mass spectrometry and cluster calculation. Sci Technol Adv Mater 5:635–645. https://doi.org/10.1016/j.stam.2004.03.017
Zhu Y-C, Bando Y, Yin L-W, Golberg D (2004) Hollow boron nitride (BN) nanocages and BN-nanocage-encapsulated nanocrystals. Chem Eur J 10:3667–3672. https://doi.org/10.1002/chem.200400002
Oku T, Nishiwaki A, Narita I (2004) Formation and structure of B28N28 clusters studied by mass spectrometry and molecular orbital calculation. Solid State Commun 130:171–173. https://doi.org/10.1016/j.ssc.2004.02.004
Oku T, Nishiwaki A, Narita I (2004) Formation and structures of B36N36 and Y@B36N36 clusters studied by high-resolution electron microscopy and mass spectrometry. J Phys Chem Solids 65:369–372. https://doi.org/10.1016/j.jpcs.2003.09.010
Oku T (2015) Hydrogen storage in boron nitride and carbon nanomaterials. Energies 8:319–337. https://doi.org/10.3390/en8010319
Beheshtian J, Peyghan AA, Bagheri Z (2012) Selective function of Al12N12 nanocage towards NO and CO molecules. Comput Mater Sci 62:71–74. https://doi.org/10.1016/j.commatsci.2012.05.041
Esrafili MD, Abdollahpour H, Saeidi N (2018) Metal-free reduction of NO over a fullerene-like boron nitride nanocluster: a mechanistic study by DFT calculations. ChemistrySelect 3:1168–1175. https://doi.org/10.1002/slct.201702812
Raad NH, Manavizadeh N, Frank I, Nadimi E (2021) Gas sensing properties of a two-mensional graphene/h-BN multi-heterostructure toward H2O, NH3 and NO2: a first principles study. Appl Surf Sci 565:150454. https://doi.org/10.1016/j.apsusc.2021.150454
Ammar HY, Badran HM, Eid KhM (2020) TM-doped B12N12 nano-cage (TM = Mn, Fe) as a sensor for CO, NO, and NH3 gases: a DFT and TD-DFT study. Mater Today Commun 25:101681. https://doi.org/10.1016/j.mtcomm.2020.101681
Shimizu T, Huang D, Yan F, Stranava M, Bartosova M, Fojtíková V, Martínková M (2015) Gaseous O2, NO, and CO in signal transduction: structure and function relationships of heme-based gas sensors and heme-redox sensors. Chem Rev 115:6491–6533. https://doi.org/10.1021/acs.chemrev.5b00018
Gao S, Khan A, Nazari M, Mirzaei H, Lup ANK, Baei MT, Chandiramouli R, Soltani A, Salehi A, Javan M, Jokar MH, Pishnamazi M, Nouri A (2021) Molecular modeling and simulation of glycine functionalized B12N12 and B16N16 nanoclusters as potential inhibitors of proinflammatory cytokines. J Mol Liq 343:117494. https://doi.org/10.1016/j.molliq.2021.117494
Cao Y, Khan A, Ghorbani F, Mirzaei H, Singla P, Balakheyli H, Soltani A, Aghaei M, Azmoodeh Z, Aarabi M, Tavassoli S (2021) Predicting adsorption behavior and anti-inflammatory activity of naproxen interacting with pure boron nitride and boron phosphide fullerene-like cages. J Mol Liq 339:116678. https://doi.org/10.1016/j.molliq.2021.116678
Hadipour NL, Peyghan AA, Soleymanabadi H (2015) J Phys Chem C 119:6398–6404. https://doi.org/10.1021/jp513019z
Silva ALP, Varela Júnior JJG (2022) Carbon monoxide interaction with B12N12 nanocage with and without an external electric field: a DFT study. J Nanopart Res 24:1. https://doi.org/10.1007/s11051-021-05382-3
Rostamoghli R, Vakili M, Banaei A, Pourbashir E, Jalalierad K (2018) Applying the B12N12 nanoparticle as the CO, CO2, H2O and NH3 sensor. Chem Rev Lett 1:31–36. https://doi.org/10.22034/crl.2018.85214
Rauf HG, Mahmood EA, Majedi S, Sofi M (2019) Adsorption behavior of the Al- and Ga-doped B12N12 nanocages on COn (n=1, 2) and HnX (n=2, 3 and X=O, N): a comparative study. Chem Rev Lett 2:140–150. https://doi.org/10.22034/crl.2020.214660.1029
Silva ALP, Silva ACA, Navis CN, Varela Júnior JJG (2021) Theoretical study of putrescine and X12Y12 (X=Al, B and Y=N, P) nanocage interactions. J Nanoparticle Res 23:108. https://doi.org/10.1007/s11051-021-05211-7
Kaviani S, Shahab S, Sheikhi M (2021) Adsorption of alprazolam drug on the B12N12 and Al12N12 nano-cages for biological applications: a DFT study. Physica E Low Dimens Syst Nanostruct 126:114473. https://doi.org/10.1016/j.physe.2020.114473
Shamim SUD, Miah MH, Hossain MR, Hasan MM, Hossain MK, Hossain MA, Ahmed F (2022) Theoretical investigation of emodin conjugated doped B12N12 nanocage by means of DFT, QTAIM and PCM analysis. Physica E Low Dimens Syst Nanostruct 136:115027. https://doi.org/10.1016/j.physe.2021.115027
Beheshtian J, Bagheri Z, Kamfiroozi M, Ahmadi A (2011) Toxic CO detection by B12N12 nanocluster. Microelectronics J 42:1400–1403. https://doi.org/10.1016/j.mejo.2011.10.010
Soltani A, Javan MB (2015) Carbon monoxide interactions with pure and doped B11XN12 (X = Mg, Ge, Ga) nano-clusters: a theoretical study. RSC Adv 5:90621–90631. https://doi.org/10.1039/C5RA12571E
Eid KhM, Ammar HY (2012) A density functional study of NO2 adsorption on perfect and defective MgO (100) and Li/MgO (100) surfaces. Appl Surf Sci 258:7689–7698. https://doi.org/10.1016/j.apsusc.2012.04.124
Esrafili MD, Asadollahi S, Heydari S (2019) A DFT study on NO reduction to N2O using Al- and P-doped hexagonal boron nitride nanosheets. J Mol Graph Model 89:41–49. https://doi.org/10.1016/j.jmgm.2019.02.013
Monks PS, Granier C, Fuzzi S et al (2009) Atmospheric composition change – global and regional air quality. Atmos Environ 43:5268–5350. https://doi.org/10.1016/j.atmosenv.2009.08.021
Santucci S, Picozzi S, Gregorio FD, Lozzi L, Cantalini C, Valentini L, Kenny JM, Delley B (2003) NO2 and CO gas adsorption on carbon nanotubes: experiment and theory. J Chem Phys 119:10904–109010. https://doi.org/10.1063/1.1619948
Beheshtian J, Peyghan AA, Bagheri Z, Kamfiroozi M (2012) Interaction of small molecules (NO, H2, N2, and CH4) with BN nanocluster surface. Struct Chem 23:1567–1572. https://doi.org/10.1007/s11224-012-9970-9
Rad AS, Ayub K (2016) Enhancement in hydrogen molecule adsorption on B12N12 nano-cluster by decoration of nickel. Int J Hydrog Energy 41:22182–22191. https://doi.org/10.1016/j.ijhydene.2016.08.158
Rahimi F, Zabaradsti A (2017) Photo-induced electron transfer process on pristine and Sc-Substituted B12N12 Nanocage as H2S Chemosensor: a fully DFT and TD-DFT study. J Inorg Organomet Polym 27:1770–1777. https://doi.org/10.1007/s10904-017-0640-7
Hussain S, Hussain R, Mehboob MY, Chatha SAS, Hussain AI, Umar A, Khan MU, Ahmed M, Adnan M, Ayub K (2020) Adsorption of phosgene gas on pristine and copper-decorated B12N12 nanocages: a comparative DFT study. ACS Omega 5:7641–7650. https://doi.org/10.1021/acsomega.0c00507
Silva ALP, Sousa NS, Varela Júnior JJG (2023) Theoretical studies with B12N12 as a toxic gas sensor: a review. J Nanoparticle Res 25:22. https://doi.org/10.1007/s11051-023-05667-9
Ammar HY, Badran HM, Eid KhM (2021) The impact of an external electric field on methanol adsorption on XB11N12 (X=B Co, Ni) nanocages: a DFT and TD-DFT study. J Phys Chem Solids 153:10033. https://doi.org/10.1016/j.jpcs.2021.110033
Silva ALP, Varela Júnior JJG (2023) Density functional theory study of Cu-modified B12N12 nanocage as a chemical sensor for carbon monoxide gas. Inorg Chem 62:1926–1934. https://doi.org/10.1021/acs.inorgchem.2c01621
Grimme S (2006) Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J Comp Chem 27:1787–1799. https://doi.org/10.1002/jcc.20495
Grimme S (2011) Density functional theory with London dispersion corrections. WIREs Comput Mol Sci 1:211–228. https://doi.org/10.1002/wcms.30
Neese F (2018) Software update: the ORCA program system, version 4.0. WIREs Comput Mol Sci 8:e1327. https://doi.org/10.1002/wcms.1327
Hossain MR, Hasan MM, Nishat M, Noor-E-Ashrafi AF, Ferdous T, Abul Hossain Md (2021) DFT and QTAIM investigations of the adsorption of chlormethine anticancer drug on the exterior surface of pristine and transition metal functionalized boron nitride fullerene. J Mol Liq 323:114627. https://doi.org/10.1016/j.molliq.2020.114627
Koopmans T (1993) Ordering of wave functions and eigenenergies to the individual electrons of an atom. Physica 1:104–113. https://doi.org/10.1016/S0031-8914(34)90011-2
Grimme S, Bannwarth C, Shushkov P (2017) A robust and accurate tight-binding quantum chemical method for structures, vibrational frequencies, and noncovalent interactions of large molecular systems parametrized for all spd-block elements (Z = 1–86). J Chem Theory Comput 13:1989–2009. https://doi.org/10.1021/acs.jctc.7b00118
Escobedo-Morales A, Tepech-Carrillo L, Bautista-Hernández A, Camacho-García JH, Cortes-Arriagada D, Chigo-Anota E (2019) Effect of chemical order in the structural stability and physicochemical properties of B12N12 fullerenes. Sci Rep 9:16521. https://doi.org/10.1038/s41598-019-52981-1
Sutradhar T, Misra A (2021) Theoretical study on the nonlinear optical property of boron nitride nanoclusters functionalized by electron donating and electron accepting groups. J Phys Chem A 125:2436–2445. https://doi.org/10.1021/acs.jpca.0c11101
Zhao Z, Li Z, Wang Q (2020) Structures, electronic and magnetic properties of transition metal atoms encapsulated in B12N12 cage. Chem Phys Lett 739:136922. https://doi.org/10.1016/j.cplett.2019.136922
Silva ALP, Silva ACA, Varela Júnior JJG (2022) Putrescine adsorption on pristine and Cu-decorated B12N12 nanocages: a density functional theory study. Comput Theor Chem 1215:113836. https://doi.org/10.1016/j.comptc.2022.113836
Baei MT (2013) Si-Doped B12N12 nanocage as an adsorbent for dissociation of N2O to N2 molecule. Heteroat Chem 24:476–481. https://doi.org/10.1002/hc.21114
Larki S, Shakerzadeh E, Anota EC, Behjatmanesh-Ardakani R (2019) The Al, Ga and Sc dopants effect on the adsorption performance of B12N12 nanocluster toward pnictogen hydrides. Chem Phys 526:110424. https://doi.org/10.1016/j.chemphys.2019.110424
Janjua MRSA (2021) Prediction and understanding: quantum chemical framework of transition metals enclosed in a B12N12 inorganic nanocluster for adsorption and removal of DDT from the environment. Inorg Chem 60:10837–10847. https://doi.org/10.1021/acs.inorgchem.1c01760
Pearson RG (1986) Absolute electronegativity and hardness correlated with molecular orbital theory. Proc Natl Acad Sci USA 83:8440–8441. https://doi.org/10.1073/pnas.83.22.8440
Parr RG, Szentpaly LV, Liu S (1999) Electrophilicity index. J Am Chem Soc 121:1922–1924. https://doi.org/10.1021/ja983494x
Cui H, Jia P, Peng X, Li P (2020) Adsorption and sensing of NO and C2H2 by S-defected SnS2 monolayer for DGA in transformer oil: a DFT study. Mater Chem Phys 249:123006. https://doi.org/10.1016/j.matchemphys.2020.123006
Celaya CA, Hernandez-Ayala LF, Zamudio FB, Vargas JA, Reina M (2021) Adsorption of melphalan anticancer drug on C24, B12N12, B12C6N6, B6C12N12 and B6C6N12 nanocages: a comparative DFT study. J Mol Liq 329:115528. https://doi.org/10.1016/j.molliq.2021.115528
Li S (2006) Semiconductor physical electronics, second ed., Springer, Berlin. https://doi.org/10.1007/0-387-37766-2
Cui H, Zhang X, Zhang G, Tang J (2019) Pd-doped MoS2 monolayer: a promising candidate for DGA in transformer oil based on DFT method. Appl Surf Sci 470:1035–1042. https://doi.org/10.1016/j.apsusc.2018.11.230
Potje-Kamloth K (2008) Semiconductor junction gas sensors. Chem Rev 108:367–399. https://doi.org/10.1021/cr0681086
Hossain MdA, Hossain MdR, Hossain MdK, Khandaker JI, Ahmed F, Ferdous T, Hossain MdA (2020) An ab initio study of the B35 boron nanocluster for application as atmospheric gas (NO, NO2, N2O, NH3) sensor. Chem Phys Lett 754:137701. https://doi.org/10.1016/j.cplett.2020.137701
Patel S, Patel P, Chodvadiya D, Som NN, Jha PK (2022) Adsorption performance of C12, B6N6 and Al6N6 nanoclusters towards hazardous gas molecules: a DFT investigation for gas sensing and removal application. J Mol Liq 352:118702. https://doi.org/10.1016/j.molliq.2022.118702
Vadalkar S, Chodvadiya D, Som NN, Vyas KN, Jha PK, Chakraborty B (2022) An Ab-initio study of the C18 nanocluster for hazardous gas sensor application. ChemistrySelect 7:e2021038741. https://doi.org/10.1002/slct.202103874
Baei MT, Peyghan AA, Bagheri Z, Tabar MB (2012) B-doping makes the carbon nanocones sensitive towards NO molecules. Phys Lett A 377:107–111. https://doi.org/10.1016/j.physleta.2012.11.006
Acknowledgements
The authors acknowledge the financial support provided by CAPES, CNPq, and FAPEMA.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Compliance with ethical standards
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
de Sousa Sousa, N., Silva, A.L.P., Silva, A.C.A. et al. Cu-modified B12N12 nanocage as a chemical sensor for nitrogen monoxide gas: a density functional theory study. J Nanopart Res 25, 248 (2023). https://doi.org/10.1007/s11051-023-05898-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-023-05898-w