Abstract
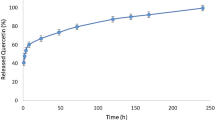
Hesperidin as a natural flavonoid has a wide range of beneficial properties such as anti-oxidant, anti-cancer, and anti-inflammatory effects. However, low water solubility and low bioavailability have limited its use in clinic. Selenium nanoparticles have attracted great interest in recent years due to their unique characteristics and anti-oxidant activates. Therefore, in this study, we synthesized hesperidin-loaded selenium nanoparticles stabilized by chitosan (Hesp-SeNPs@CS) and investigated its protective effects against glutamate-induced toxicity in PC12 cells. The physicochemical properties of the nanoparticles were evaluated using various techniques. The MTT and intracellular reactive oxygen species (ROS) assay were performed to evaluate the cell viability and ROS levels, respectively. In addition, flow cytometry was used to determine the cell cycle arrest and apoptosis rate in PC12 cells. Our results showed that, Hesp-SeNPs@CS nanoparticles have a 210.6 nm diameter and a negative zeta potential of -15.9 mV. The results of MTT assay revealed that glutamate significantly decreased cell viability by about 50% at a concentration of 20 mM. However, hesperidin at the concentration of 62.5 µM and Hesp-SeNPs@CS at the concentrations of 62.5 and 125 μg/ml significantly decreased the glutamate-induced cell toxicity, sub-G1 cell cycle arrest and apoptosis rate in PC12 cells. In addition, in the groups treated with hesperidin and Hesp-SeNPs@CS, the intracellular ROS induced by glutamate showed a significant decrease compared to glutamate-treated group. Taken together, these results revealed that Hesp-SeNPs@CS has protective effects against glutamate-induced cytotoxicity and oxidative stress damage in PC12 cells that merit further investigation.
Graphical abstract







Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
- AD:
-
Alzheimer
- CNS:
-
Central nervous system
- DCFH-DA:
-
2′,7′-Dichlorodihydrofluorescein diacetate
- DMSO:
-
Dimethyl sulfoxide
- EDX:
-
Energy dispersive X-ray spectrum
- FBS:
-
Fetal bovine serum
- FESEM:
-
Field emission scanning electron microscope
- FTIR:
-
Fourier transform infrared
- Glu:
-
Glutamate
- GPx:
-
Glutathione peroxidase
- Hesp:
-
Hesperidin
- Hesp-SeNPs@CS:
-
Hesperidin-loaded selenium nanoparticles stabilized by chitosan
- HD:
-
Huntington
- NAC:
-
N-acetyl cysteine
- NP:
-
Nanoparticle
- PD:
-
Parkinson
- ROS:
-
Reactive oxygen species
- PI:
-
Propidium iodide
- rpm:
-
Round per minute
- Se:
-
Selenium
References
Choi DW (1988) Glutamate neurotoxicity and diseases of the nervous system. Neuron 1:623–634
Coyle JT, Puttfarcken P (1993) Oxidative stress, glutamate, and neurodegenerative disorders. Science 262:689–695
Guo JD, Zhao X, Li Y, Li GR, Liu XL (2018) Damage to dopaminergic neurons by oxidative stress in Parkinson’s disease (Review). Int J Mol Med 41:1817–1825
Oprea D, Sanz CG, Barsan MM, Enache TA (2022) PC-12 cell line as a neuronal cell model for biosensing applications. Biosensors 12:500
Kawakami Z, Kanno H, Ikarashi Y, Kase Y (2011) Yokukansan, a kampo medicine, protects against glutamate cytotoxicity due to oxidative stress in PC12 cells. J Ethnopharmacol 134:74–81
Li N, Liu B, Dluzen DE, Jin Y (2007) Protective effects of ginsenoside Rg2 against glutamate-induced neurotoxicity in PC12 cells. J Ethnopharmacol 111:458–463
Atlante A, Calissano P, Bobba A, Giannattasio S, Marra E, Passarella S (2001) Glutamate neurotoxicity, oxidative stress and mitochondria. FEBS Lett 497:1–5
Lu C-W, Lin T-Y, Wang S-J (2010) Memantine depresses glutamate release through inhibition of voltage-dependent Ca2+ entry and protein kinase C in rat cerebral cortex nerve terminals: an NMDA receptor-independent mechanism. Neurochem Int 57:168–176
Penugonda S, Mare S, Goldstein G, Banks WA, Ercal N (2005) Effects of N-acetylcysteine amide (NACA), a novel thiol antioxidant against glutamate-induced cytotoxicity in neuronal cell line PC12. Brain Res 1056:132–138
Manthey JA, Grohmann K, Guthrie N (2001) Biological properties of citrus flavonoids pertaining to cancer and inflammation. Curr Med Chem 8:135–153
Nogata Y, Sakamoto K, Shiratsuchi H, Ishii T, Yano M, Ohta H (2006) Flavonoid composition of fruit tissues of citrus species. Biosci Biotechnol Biochem 70:178–192
Parhiz H, Roohbakhsh A, Soltani F, Rezaee R, Iranshahi M (2015) Antioxidant and anti-inflammatory properties of the citrus flavonoids hesperidin and hesperetin: an updated review of their molecular mechanisms and experimental models. Phytother Res 29:323–331
Park H, Kim M-J, Ha E, Chung J-H (2008) Apoptotic effect of hesperidin through caspase3 activation in human colon cancer cells, SNU-C4. Phytomedicine 15:147–151
Kim J, Wie MB, Ahn M, Tanaka A, Matsuda H, Shin T (2019) Benefits of hesperidin in central nervous system disorders: a review. Anat Cell Biol 52:369–377
Majumdar S, Srirangam R (2009) Solubility, stability, physicochemical characteristics and in vitro ocular tissue permeability of hesperidin: a natural bioflavonoid. Pharm Res 26:1217–1225
Moghimi SM, Hunter AC, Murray JC (2005) Nanomedicine: current status and future prospects. FASEB J 19:311–330
Ansar S, Abudawood M, Alaraj ASA, Hamed SS (2018) Hesperidin alleviates zinc oxide nanoparticle induced hepatotoxicity and oxidative stress. BMC Pharmacol Toxicol 19:65
Sulaiman GM, Waheeb HM, Jabir MS, Khazaal SH, Dewir YH, Naidoo Y (2020) Hesperidin loaded on gold nanoparticles as a drug delivery system for a successful biocompatible, anti-cancer, anti-inflammatory and phagocytosis inducer model. Sci Rep 10:9362
Khiralla GM, El-Deeb BA (2015) Antimicrobial and antibiofilm effects of selenium nanoparticles on some foodborne pathogens. LWT-Food Science and Technology 63:1001–1007
Li Y, Li X, Zheng W, Fan C, Zhang Y, Chen T (2013) Functionalized selenium nanoparticles with nephroprotective activity, the important roles of ROS-mediated signaling pathways. J Mater Chem B 1:6365–6372
Shakibaie M, Khorramizadeh MR, Faramarzi MA, Sabzevari O, Shahverdi AR (2010) Biosynthesis and recovery of selenium nanoparticles and the effects on matrix metalloproteinase-2 expression. Biotechnol Appl Biochem 56:7–15
Jameel MS, Aziz AA, Dheyab MA (2020) Green synthesis: Proposed mechanism and factors influencing the synthesis of platinum nanoparticles. Green Processes Synth 9:386–398
Rónavári A, Igaz N, Adamecz DI, Szerencsés B, Molnar C, Kónya Z et al (2021) Green silver and gold nanoparticles: biological synthesis approaches and potentials for biomedical applications. Molecules 26:844
Herdiana Y, Wathoni N, Shamsuddin S, Muchtaridi M (2022) Drug release study of the chitosan-based nanoparticles. Heliyon 8:e08674
Zhang E, Xing R, Liu S, Qin Y, Li K, Li P (2019) Advances in chitosan-based nanoparticles for oncotherapy. Carbohydr Polym 222:115004
Luesakul U, Puthong S, Neamati N, Muangsin N (2018) pH-responsive selenium nanoparticles stabilized by folate-chitosan delivering doxorubicin for overcoming drug-resistant cancer cells. Carbohydr Polym 181:841–850
Jangde R, Elhassan GO, Khute S, Singh D, Singh M, Sahu RK et al (2022) Hesperidin-loaded lipid polymer hybrid nanoparticles for topical delivery of bioactive drugs. Pharmaceuticals (Basel) 15:211
Memari F, Mirzavi F, Jalili-Nik M, Afshari AR, Ghorbani A, Soukhtanloo M (2022) Tumor-inhibitory effects of zerumbone against HT-29 human colorectal cancer cells. Int J Toxicol 41:402–411
Mirzavi F, Barati M, Vakili-Ghartavol R, Roshan MK, Mashreghi M, Soukhtanloo M et al (2022) Pegylated liposomal encapsulation improves the antitumor efficacy of combretastatin A4 in murine 4T1 triple-negative breast cancer model. Int J Pharm 613:121396
Bemidinezhad A, Mirzavi F, Gholamhosseinian H, Gheybi F, Soukhtanloo M (2023) Gold-containing liposomes and glucose-coated gold nanoparticles enhances the radiosensitivity of B16F0 melanoma cells via increasing apoptosis and ROS production. Life Sci 318:121495
Balakrishnan K, Casimeer SC, Ghidan AY, Ghethan FY, Venkatachalam K, Singaravelu A (2021) Bioformulated hesperidin-loaded PLGA nanoparticles counteract the mitochondrial-mediated intrinsic apoptotic pathway in cancer cells. J Inorg Organomet Polym Mater 31:331–343
Queiroz MF, Teodosio Melo KR, Sabry DA, Sassaki GL, Rocha HAO (2014) Does the use of chitosan contribute to oxalate kidney stone formation? Mar Drugs 13:141–158
Bhatia P, Pandey S, Prakash R, Nagaraja TP (2014) Enhanced anti-oxidant activity as a function of selenium hyperaccumulation in Agaricus bisporus cultivated on Se-rich Agri-residues. J Biol Act Prod Nat 4:354–364
Sansone F, Rossi A, Del Gaudio P, De Simone F, Aquino RP, Lauro MR (2009) Hesperidin gastroresistant microparticles by spray-drying: preparation, characterization, and dissolution profiles. AAPS PharmSciTech 10:391–401
Rana A, Yadav K, Jagadevan S (2020) A comprehensive review on green synthesis of nature-inspired metal nanoparticles: mechanism, application and toxicity. J Clean Prod 272:122880
Rajalakshmi S, Vijayakumar S, Praseetha P (2019) Neuroprotective behaviour of Phyllanthus emblica (L) on human neural cell lineage (PC12) against glutamate-induced cytotoxicity. Gene Rep 17:100545
Yu D, Duan Y, Bao Y, Wei C, An L (2005) Isoflavonoids from Astragalus mongholicus protect PC12 cells from toxicity induced by L-glutamate. J Ethnopharmacol 98:89–94
Nishizawa Y (2001) Glutamate release and neuronal damage in ischemia. Life Sci 69:369–381
Chen B, Zhao J, Zhang R, Zhang L, Zhang Q, Yang H et al (2022) Neuroprotective effects of natural compounds on neurotoxin-induced oxidative stress and cell apoptosis. Nutr Neurosci 25:1078–1099
Zheng YZ, Deng G, Guo R, Fu ZM, Chen DF (2019) Theoretical insight into the antioxidative activity of isoflavonoid: the effect of the C2=C3 double bond. Phytochemistry 166:112075
Hwang SL, Yen GC (2008) Neuroprotective effects of the citrus flavanones against H2O2-induced cytotoxicity in PC12 cells. J Agric Food Chem 56:859–864
Antunes MS, Goes AT, Boeira SP, Prigol M, Jesse CR (2014) Protective effect of hesperidin in a model of Parkinson’s disease induced by 6-hydroxydopamine in aged mice. Nutrition (Burbank, Los Angeles County, Calif.) 30:1415–1422
Justin-Thenmozhi A, Dhivya Bharathi M, Kiruthika R, Manivasagam T, Borah A, Essa MM (2018) Attenuation of aluminum chloride-induced neuroinflammation and caspase activation through the AKT/GSK-3beta pathway by hesperidin in wistar rats. Neurotox Res 34:463–476
Wang D, Liu L, Zhu X, Wu W, Wang Y (2014) Hesperidin alleviates cognitive impairment, mitochondrial dysfunction and oxidative stress in a mouse model of Alzheimer’s disease. Cell Mol Neurobiol 34:1209–1221
Hesperidin PK (2022) A review on extraction methods, stability and biological activities. Nutrients. 2022/06//; 14(12):[2387 p.]
Buacheen P, Chaipuang A, Karinchai J, Nuchuchua O, Imsumran A, Wongnoppavich A et al (2023) Stabilization of antioxidant and anti-inflammatory activities of Nano-Selenium using Anoectochilus Burmannicus extract as a potential novel functional ingredient. Nutrients 15:1018
Shi XD, Tian YQ, Wu JL, Wang SY (2021) Synthesis, characterization, and biological activity of selenium nanoparticles conjugated with polysaccharides. Crit Rev Food Sci Nutr 61:2225–2236
Ceballos-Picot I, Merad-Boudia M, Nicole A, Thevenin M, Hellier G, Legrain S et al (1996) Peripheral antioxidant enzyme activities and selenium in elderly subjects and in dementia of Alzheimer’s type–place of the extracellular glutathione peroxidase. Free Radical Biol Med 20:579–587
Zambonino MC, Quizhpe EM, Jaramillo FE, Rahman A, Santiago Vispo N, Jeffryes C et al (2021) Green synthesis of Selenium and Tellurium nanoparticles: current trends, biological properties and biomedical applications. Int J Mol Sci 22:989
Abd-Eltawab Tammam A, Khalaf AAA, Zaki AR, Mansour Khalifa M, Ibrahim MA, Mekkawy AM et al (2022) Hesperidin protects rats’ liver and kidney from oxidative damage and physiological disruption induced by nickel oxide nanoparticles. Front Physiol 13:912625
Khiralla G, Elhariry H, Selim SM (2020) Chitosan-stabilized selenium nanoparticles attenuate acrylamide-induced brain injury in rats. J Food Biochem 44:e13413
Rao S, Lin Y, Lin R, Liu J, Wang H, Hu W et al (2022) Traditional Chinese medicine active ingredients-based selenium nanoparticles regulate antioxidant selenoproteins for spinal cord injury treatment. J Nanobiotechnol 20:1–15
Funding
The authors would like to thank the Mashhad University of Medical Sciences for their financial support [Grant No. 991653].
Author information
Authors and Affiliations
Contributions
Zohreh Najafi: Writing-original draft, Methodology. Elham Einafshar: Writing-original draft, Investigation, Validation. Farshad Mirzavi: Writing- review & editing. Hamed Amiri: Investigation. Mohammad Jalili-Nik: Methodology, Formal analysis. Mohammad Soukhtanloo: Conceptualization, Supervision. All authors have read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethical approval
This declaration is “not applicable”.
Conflict of interest
No potential conflict of interest was reported by the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Zohreh Najafi and Elham Einafshar contributed equally as first authors.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Najafi, Z., Einafshar, E., Mirzavi, F. et al. Protective effect of hesperidin-loaded selenium nanoparticles stabilized by chitosan on glutamate-induced toxicity in PC12 cells. J Nanopart Res 25, 178 (2023). https://doi.org/10.1007/s11051-023-05828-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-023-05828-w