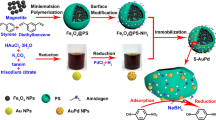
Abstract
Although the immobilization of gold nanoparticles (Au NPs) on the support is a conventional method for preventing them from aggregation and improving their separability at the cost of activity loss, herein, we developed a facile method to prepare supported Au NPs with the higher catalytic activity and better separability due to the selective adsorption of its functional surface. Firstly, the multi-functional carriers (amino-modified magnetic microspheres) were synthesized to immobilize Au NPs. Depending on its surface adsorption towards the reactant (p-nitrophenol), this carrier could greatly improve the mass transfer between p-nitrophenol (4-NP) and Au NPs resulting in the improvement of catalytic activity of supported Au NPs. The catalytic activity of supported Au NPs is increased more than 6.65 times compared with that of isolated Au NPs. Then, the effects of the particle size and supporting density of Au NPs on the catalytic activity were also investigated. Turnover frequency value of supported Au NPs (3.8 nm) reaches 16,000 h−1 when its surface density is controlled to 2211 μg g−1. Furthermore, the catalyst of Au/Fe3O4@PS-NH2 showed excellent catalytic activity when various nitrobenzene derivatives were employed as substrates. Remarkably, these supported Au NPs could be easily isolated by magnetic separation in 30 s. This catalyst could be recycled for 45 times without any loss in catalytic activity. The high catalytic activity and easy separability of this supported Au NPs make it much potential in large-scale application.

A magnetic carrier was prepared and its surface was modified with amino groups. Depending on the selective adsorption for 4-NP, the catalytic activity of supported Au NPs was greatly improved. This supported Au NPs could be easily isolated by magnetic separation and recycled for 45 times without any loss in catalytic activity. Thus, the higher catalytic activity and easier separation of supported Au NPs are smoothly combined together.








Similar content being viewed by others
References
Aditya T, Pal A, Pal T (2015) Nitroarene reduction: a trusted model reaction to test nanoparticle catalysts. Chem Commun 51:9410–9431
Bus E, Prins R, van Bokhoven JA (2007) Origin of the cluster-size effect in the hydrogenation of cinnamaldehyde over supported Au catalysts. Catal Commun 8:1397–1402
Cho A, Byun S, Kim BM (2018) AuPd-Fe3O4 nanoparticle catalysts for highly selective, one-pot cascade nitro-reduction and reductive amination. Adv Synth Catal 360:1253–1261
Dalpozzo R (2015) Magnetic nanoparticle supports for asymmetric catalysts. Green Chem 17:3671–3686
Fan CM, Zhang LF, Wang SS, Wang DH, Lu LQ, Xu AW (2012) Novel CeO2 yolk–shell structures loaded with tiny Au nanoparticles for superior catalytic reduction of p-nitrophenol. Nanoscale 4:6835–6840
Fang J, Li JG, Zhang B, Yuan X, Asakura H, Tanaka T, Teramura K, Xie JP, Yan N (2015) The support effect on the size and catalytic activity of thiolated Au25 nanoclusters as precatalysts. Nanoscale 7:6325–6333
Golabiewska A, Malankowska A, Jarek M, Lisowski W, Nowaczyk G, Jurga S, Adriana Z-M (2016) The effect of gold shape and size on the properties and visible light-induced photoactivity of Au-TiO2. Appl Catal B-Environ 196:27–40
Gu H, Wang JN, Ji YC, Wang ZQ, Chen W, Xue G (2013) Facile and controllable fabrication of gold nanoparticles-immobilized hollow silica particles and their high catalytic activity. J Mater Chem A 1:12471–12477
Han J, Li LY, Guo R (2010) Novel approach to controllable synthesis of gold nanoparticles supported on polyaniline nanofibers. Macromolecules 43:10636–10644
Handa S, Wang Y, Gallou F, Lipshutz BH (2015) Sustainable Fe-ppm Pd nanoparticle catalysis of Suzuki-Miyaura cross-couplings in water, vol 6252, pp 1087–1091
Haruta M (2004) Gold as a novel catalyst in the 21st century: preparation, working mechanism and applications. Gold Bull 37:27–36
Heinz H, Pramanik C, Heinz O, Ding YF, Mishra RK, Marchon D, Flatt RJ, Estrela-Lopis I, Llop J, Moya S, Ziolo RF (2017) Nanoparticle decoration with surfactants: molecular interactions, assembly, and applications. Sur Sci Rep 72:1–58
Kruss S, Srot V, van Aken PA, Spatz JP (2011) Au-Ag hybrid nanoparticle patterns of tunable size and density on glass and polymeric supports. Langmuir 28:1562–1568
Lei M, Wu W, Yang SL, Zhang XG, Xing Z, Ren F, Xiao XH, Jiang CZ (2016) Design of enhanced catalysts by coupling of noble metals (Au, Ag) with semiconductor SnO2 for catalytic reduction of 4-nitrophenol. Part Part Syst Charact 33:212–220
Li ML, Chen GF (2013) Revisiting catalytic model reaction p-nitrophenol/NaBH 4 using metallic nanoparticles coated on polymeric spheres. Nanoscale 5:11919–11927
Li SJ, Bao D, Shi MM, Wulan BR, Yan JM, Jiang Q (2017a) Amorphizing of Au nanoparticles by CeOx–RGO hybrid support towards highly efficient electrocatalyst for N2 reduction under ambient conditions. Adv Mater 29:1700001
Li CJ, Wang Z, Li Q, Peng LY, Zhang WQ, Zhang YX, Qian H (2017b) Improving catalytic activity of supported Au nanoparticles depending on its density. Mol Catal 427:18–24
Li YW, Chen YX, House SD, Zhao S, Wahab Z, Yang JC, Jin RC (2018a) Interface engineering of gold nanoclusters for CO oxidation catalysis. ACS Appl Mater Inter 10:29425–29434
Li DD, Zhang JW, Cai C (2018b) Chemoselective hydrogenation of nitroarenes catalyzed by cellulose supported Pd NPs. Catal Commun 103:47–50
Liang SZ, Jasinski J, Hammond GB, Xu B (2015) Supported gold nanoparticle-catalyzed hydration of alkynes under basic conditions. Org Lett 17:162–165
Lin C, Tao K, Hua D, Ma Z, Zhou S (2013) Size effect of gold nanoparticles in catalytic reduction of p-nitrophenol with NaBH4. Molecules 18:12609–12620
Ma ML, Yang YY, Li WT, Feng RJ, Li ZW, Lyu P, Ma Y (2018) Gold nanoparticles supported by amino groups on the surface of magnetite microspheres for the catalytic reduction of 4-nitrophenol. J Mater Sci 54:323–334
Marelli M, Evangelisti C, Diamanti MV, Dal Santo V, Pedeferri MP, Bianchi CL, Schiavi L, Strini A (2016) TiO2 nanotubes arrays loaded with ligand-free Au nanoparticles: enhancement in photocatalytic activity. ACS Appl Mater Inter 8:31051–31058
Nieto-Ortega B, Bürgi T (2018) Vibrational properties of thiolate-protected gold nanoclusters. Acc Chem Res 51:2811–2819
Nutt MO, Heck KN, Alvarez P, Wong MS (2006) Improved Pd-on-Au bimetallic nanoparticle catalysts for aqueous-phase trichloroethene hydrodechlorination. Appl Catal B-Environ 69:115–125
Pan BC, Chen XQ, Pan BJ, Zhang WM, Zhang X, Zhang QX (2006) Preparation of an aminated macroreticular resin adsorbent and its adsorption of p-nitrophenol from water. J Hazard Mater 137:1236–1240
Shin HS, Huh S (2012) Au/Au@ polythiophene core/shell nanospheres for heterogeneous catalysis of nitroarenes. ACS Appl Mater Interfaces 4:6324–6331
Tang HL, Wei JK, Liu F, Qiao BT, Pan XL, Li L, Liu JY, Wang JH, Zhang T (2016) Strong metal-support interactions between gold nanoparticles and nonoxides. J Am Chem Soc 138:56–59
Tuo Y, Liu GF, Dong B, Zhou JT, Wang AJ, Wang J, Jin RF, Lv H, Dou ZO, Huang WY (2015) Microbial synthesis of Pd/Fe3O4, Au/Fe3O4 and PdAu/Fe3O4 nanocomposites for catalytic reduction of nitroaromatic compounds. Sci Rep 5:13515
Udawatte N, Lee M, Kim J, Lee D (2011) Well-defined Au/ZnO nanoparticle composites exhibiting enhanced photocatalytic activities. ACS Appl Mater Inter 3:4531–4538
Wang Z, Yu Y, Zhang YX, Li SZ, Qian H, Lin ZY (2015) A magnetically separable palladium catalyst containing a bulky N-heterocyclic carbene ligand for the Suzuki-Miyaura reaction. Green Chem 17:413–420
Wang Y, Li L, Wang CG, Wang TT (2016a) Facile approach to synthesize uniform Au@mesoporous SnO2 yolk–shell nanoparticles and their excellent catalytic activity in 4-nitrophenol reduction. J Nanopart Res 18(2)
Wang LK, Tang ZH, Yan W, Yang HY, Wang QN, Chen SW (2016b) Porous carbon-supported gold nanoparticles for oxygen reduction reaction: effects of nanoparticle size. ACS Appl Mater Interfaces 8:20635–20641
Wei F, Cai XY, Nie JQ, Wang FY, Lu CF, Yang GC, Chen ZX, Ma C, Zhang YX (2018) A 1,2,3-triazolyl based conjugated microporous polymer for sensitive detection of p-nitroaniline and Au nanoparticle immobilization. Polym Chem 9:3832–3839
Woo H, Park KH (2014) Hybrid Au nanoparticles on Fe3O4@ polymer as efficient catalyst for reduction of 4-nitrophenol. Catal Commun 46:133–137
Xu QL, Lei WY, Li XY, Qi XY, Yu JG, Liu G, Wang JL, Zhang PY (2014) Efficient removal of formaldehyde by nanosized gold on well-defined CeO2 nanorods at room temperature. Environ Sci Technol 48:9702–9708
Yang WX, Liu XJ, Yue XY, Jia JB, Guo SJ (2015) Bamboo-like carbon nanotube/Fe3C nanoparticle hybrids and their highly efficient catalysis for oxygen reduction. J Am Chem Soc 137:1436–1439
Zhang FW, Liu N, Zhao P, Sun J, Wang P, Ding W, Liu JT, Jin J, Ma JT (2012) Gold on amine-functionalized magnetic nanoparticles: a novel and efficient catalyst for hydrogenation reactions. Appl Surf Sci 263:471–475
Zhao JB, Jin RC (2018) Heterogeneous catalysis by gold and gold-based bimetal nanoclusters. Nano Today 18:86–102
Zhao PX, Feng XW, Huang DS, Yang GY, Astruc D (2015) Basic concepts and recent advances in nitrophenol reduction by gold-and other transition metal nanoparticles. Coord Chem Rev 287:114–136
Funding
This research is supported by the program of the Special Project on the Integration of Industry, Education and Research of Fujian Province (2017H6011); the Graphene Powder & Composite Research Center of Fujian Province (2017H2001); and the opening project of Key Laboratory of Ecological Environment and Information Atlas in Fujian Provincial University.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chen, J., Li, C., Sun, W. et al. High catalytic activity of supported Au nanoparticles assisted with the surface selective adsorption. J Nanopart Res 21, 146 (2019). https://doi.org/10.1007/s11051-019-4585-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-019-4585-3