Abstract
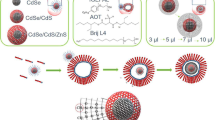
The cytotoxicity of cadmium-containing quantum dots (QDs) is reportedly caused by released Cd2+ as well as other factors such as ligand, size, and surface modification. The tolerated concentration of QDs therefore deviates from ~ 1 to 1000 nM. However, the concentration of Cd2+ released from QDs has seldom been correctly and systematically measured. We prepared highly emitting silica capsules with incorporated multiple CdSe/ZnS QDs through a sol-gel-derived wet method. The concentration of released Cd2+ in buffer solution was measured as a function of the preparation conditions of the capsules. When the shell thickness of the capsule was 15 nm, the release was effectively suppressed compared with a capsule shell thickness of 10 nm. Heat treatment at 40 °C further suppressed the leakage. When the silicon alkoxide hydrolysis time was increased from 3 to 15 h, and the surface was modified with COOH groups, the leakage reached a minimum of ~ 0.01 ppb for a QD concentration of 10 nM after 15 h of dispersion. This was two orders of magnitude less than polymer-coated analogues with the same surface functional groups. These silica capsules did not show any toxicity to culture cells, whereas the polymer-coated ones showed high toxicity with the same QD concentration. Silica capsules are therefore not only the expected ideal, but show significantly superior performance when correctly prepared for biomedical application with CdSe/ZnS QDs, owing to their strong suppression of Cd2+ leakage into solution.




Similar content being viewed by others
References
Ando M, Li CN, Murase N (2004) Photoluminescence properties and zeta potential of water-dispersible CdTe nanocrystals. Quantum Dots, Nanoparticles and Nanowires 789:123–128
Ando M, Horie M, Akazawa-Ogawa Y, Hagihara Y, Murase N, Shigeri Y (2016) Cytotoxicity of CdSe-based quantum dots incorporated in glass nanoparticles evaluated using human keratinocyte HaCaT cells. Biosci Biotechnol Biochem 80:210–213
Bradburne CE, Delehanty JB, Gemmill KB, Mei BC, Mattoussi H, Susumu K, Blanco-Canosa JB, Dawson PE, Medintz IL (2013) Cytotoxicity of quantum dots used for in vitro cellular labeling: role of QD surface ligand, delivery modality, cell type, and direct comparison to organic fluorophores. Bioconjug Chem 24:1570–1583
Brinker CJ, Scherer GW (1990) Sol-gel science: the physics and chemistry of sol-gel processing. Academic Press, Cambridge, Massachusetts
Chan Y, Zimmer JP, Stroh M, Steckel JS, Jain RK, Bawendi MG (2004) Incorporation of luminescent nanocrystals into monodisperse core–shell silica microspheres. Adv Mater 16:2092–2097
Chiang YD, Lian HY, Leo SY, Wang SG, Yamauchi Y, KC-W W (2011) Controlling particle size and structural properties of mesoporous silica nanoparticles using the Taguchi method. J Phys Chem C 115:13158–13165
Chithrani BD, Ghazani AA, Chan WCW (2006) Determining the size and shape dependence of gold nanoparticle uptake into mammalian cells. Nano Lett 6:662–668
Derfus AM, Chan WCW, Bhatia SN (2004) Probing the cytotoxicity of semiconductor quantum dots. Nano Lett 4:11–18
Francis JE, Mason D, Lévy R (2017) Evaluation of quantum dot conjugated antibodies for immunofluorescent labelling of cellular targets. Beilstein J Nanotechnol 8:1238–1249
Gerion D, Pinaud F, Williams SC, Parak WJ, Zanche D, Weiss S, Alivisatos AP (2001) Synthesis and properties of biocompatible water-soluble silica-coated CdSe/ZnS semiconductor quantum dots. J Phys Chem B 105:8861–8871
Hoshino A, Fujioka K, Oku T, Suga M, Sasaki YF, Ohta T, Yasuhara M, Suzuki K, Yamamoto K (2004) Physicochemical properties and cellular toxicity of nanocrystal quantum dots depend on their surface modification. Nano Lett 4:2163–2169
Huang H, Bodnarchuk MI, Kershaw SV, Kovalenko MV, Rogach AL (2017) Lead halide perovskite nanocrystals in the research spotlight: stability and defect tolerance. ACS Energy Lett 2:2071–2083
Koole R, Schooneveld MM, Hilhorst J, Donegá CM, Hart DC, Blaaderen A, Vanmaekelbergh D, Meijerink A (2008) On the incorporation mechanism of hydrophobic quantum dots in silica spheres by a reverse microemulsion method. Chem Mater 20:2503–2512
Lee J, Sundar VC, Heine JR, Bawendi MG, Jensen KF (2000) Full color emission from II–VI semiconductor quantum dot–polymer composites. Adv Mater 12:1102–1105
Li CL, Murase N (2005) Surfactant-dependent photoluminescence of CdTe nanocrystals in aqueous solution. Chem Lett 34:92–93
Li CL, Ando M, Enomoto H, Murase N (2008) Highly luminescent water-soluble InP/ZnS nanocrystals prepared via reactive phase transfer and photochemical processing. J Phys Chem C 112:20190–20199
Li CL, Hosokawa C, Suzuki M, Taguchi T, Murase N (2018) Preparation and biomedical applications of bright robust silica nanocapsules with multiple incorporated InP/ZnS quantum dots. New J Chem 42:18951–18960
Murase N (2010) Quantum dot-core silica glass-shell nanomaterials: synthesis, characterization and potential biomedical applications. Semiconductor nanomaterials, Chapter 11, Vol 6. In: Kumar C (ed) Nanomaterials for the life sciences. Wiley-VCH, Weinheim, pp 393–427
Murase N, Yang P (2009) Anomalous photoluminescence in silica-coated semiconductor nanocrystals after feat treatment. Small 5:800–803
Murase N, Yazawa T (2001) Partially reduced cuprous oxide nanoparticles formed in porous glass reaction fields. J Am Ceram Soc 84:2269–2273
Nooney RI, Thirunavukkarasu D, Chen Y, Josephs R, Ostafin AE (2002) Synthesis of nanoscale mesoporous silica spheres with controlled particle size. Chem Mater 14:4721–4728
Oh N, Park J-H (2014) Endocytosis and exocytosis of nanoparticles in mammalian cells. Int J Nanomedicine 9(Suppl 1):51–63
Oh E, Li R, Nel A, Gemill KB, Bilal M, Cohen Y, Medintz IL (2016) Meta-analysis of cellular toxicity for cadmium-containing quantum dots. Nat Nanotech 11:479–486
Park Y-H, Bae HC, Jang Y (2013) Effect of the size and surface charge of silica nanoparticles on cutaneous toxicity. Mol Cell Toxicol 9:67–74
Quarta A, Ragusa A, Deka S, Tortiglione C, Tino A, Cingolani R, Pellegrino T (2009) Bioconjugation of rod-shaped fluorescent nanocrystals for efficient targeted cell labeling. Langmuir 25:12614–12622
Ruan J, Wang K, Song H, Xu X, Ji J, Cui D (2011) Biocompatibility of hydrophilic silica-coated CdTe quantum dots and magnetic nanoparticles. Nanoscale Res Lett 6:299
Selvan ST, Tan TT, Ying JY (2005) Robust, non-cytotoxic, silica-coated CdSe quantum dots with efficient photoluminescence. Adv Mater 17:1620–1625
Su Y, Hub M, Fan C, He Y, Li Q, Li W, Wang L-h, Shen P, Huang Q (2010) The cytotoxicity of CdTe quantum dots and the relative contributions from released cadmium ions and nanoparticle properties. Biomaterials 31:4829–4834
Tang Y, Han S, Liu H, Chen X, Huang L, Li X, Zhang J (2013) The role of surface chemistry in determining in vivo biodistribution and toxicity of CdSe/ZnS core-shell quantum dots. Biomaterials 34:8741–8755
Tolnai G, Agod A, Kabai-Faix M, Kovács AL, Ramsden JJ, Hórvolgyi Z (2003) Evidence for secondary minimum flocculation of Stöber silica nanoparticles at the air-water interface: film balance investigations and computer simulations. J Phys Chem B 107:11109–11116
Tsuboi S, Yamada S, Nakane Y, Sakata T, Yasuda H, Jin T (2018) Water-soluble near-infrared fluorophores emitting over 1000 nm and their application to in vivo imaging in the second optical window (1000–1400 nm). ECS J Solid State Sci Technol 7:R3093–R3101
Tsunoda S (2011) Transdermal penetration and biodistribution of nanomaterials and their acute toxicity in vivo. Yakugaku Zasshi 131:203–207
Xu M, Deng G, Liu S, Chen S, Cui D, Yang L, Wang Q (2010) Free cadmium ions released from CdTe-based nanoparticles and their cytotoxicity on Phaeodactylum tricornutum. Metallomics 2:469–473
Yang P, Murase N (2010) Size-tunable highly luminescent SiO2 particles impregnated with number-adjusted CdTe nanocrystals. ChemPhysChem 11:815–821
Yang P, Murase N, Suzuki M, Hosokawa C, Kawasaki K, Kato T, Taguchi T (2010) Bright, non-blinking, and less-cytotoxic SiO2 beads with multiple CdSe/ZnS nanocrystals. Chem Commun 46:4595–4597
Yang P, Ando M, Taguchi T, Murase N (2011) Highly luminescent CdSe/CdxZn1–xS quantum dots with narrow spectrum and widely tunable wavelength. J Phys Chem C 115:14455–11460
Zhang Y, Wang M, Zheng Y-G, Tan H, Hsu BY-W, Yang Z-C, Wong SY, Chang AY-C, Choolani M, Li X, Wang J (2013) PEOlated micelle/silica as dual-layer protection of quantum dots for stable and targeted bioimaging. Chem Mater 25:2976–2985
Acknowledgements
The authors thank Dr. Takeyuki Uchida in AIST for the electron microscopy. This work was supported in part by CREST from JST, JSPS KAKENHI Grant Number JP24550242, and the Supporting Industry Program sponsored by METI.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendices
Appendices
Cytotoxicity experiment
Cells (A549 and HaCaT) were incubated with 0.5 mg/ml MTT (Nacalai Tesque, Inc.) at 37 °C for 2 h. Isopropyl alcohol containing 40 mM HCl was added to the culture medium (3:2, by volume) and the cells were mixed by pipette until the formazan was completely dissolved. The optical density of the formazan was measured at 570 nm by using a Multiskan Ascent plate reader (Thermo Labsystems, Helsinki, Finland). In the LDH assays, LDH release was measured with a tetrazoliumsalt by using a Cytotoxicity Detection KitPLUS (LDH) (Roche Diagnostics GmbH, Mannheim, Germany) according to the manufacturer’s protocol. The amount of formazan salt that formed was measured at 492 nm by using the Multiskan Ascent plate reader. The maximum amount of released LDH was determined by incubating the cells with a lysis solution provided in the kit. The cytotoxicity was calculated as follows: cytotoxicity (%) = (experimental value - low control)/(high control - low control) × 100. The low control, which refers to spontaneous LDH release, was determined as the LDH released from untreated normal cells. The high control, which refers to the maximum LDH release, was determined as the LDH released from cells that were lysed by surfactant treatment.
Concentration of released Cd2+ and measurement conditions in Xu M et al., Metallomics, 2, 469–473 (2010)
Based on the PL wavelength (slightly shorter than 550 nm) of the TGA-capped CdTe QDs, their size is estimated to be 2.6 nm [A]. These QDs therefore contain 135 Cd atoms each when the number of Cd and Te atoms is the same. Upon dialysis, a 2-mL solution of QDs (Cd, 0.1 g/L) was dialyzed against 400 mL of PBS solution. This volume of QD solution contains 13.2 nmol of QDs.
When the concentration increment of Cd2+ in dialysis solution is 1 ng/mL/mg/h in their measurement, we understand that 1 ng of Cd2+ is released per hour into 1 mL of dialysis solution from the QD solution containing 1 mg of Cd atoms.
When 1 mg of Cd atoms is in 2 mL of solution, 66 nmol of QDs are dispersed therein (the concentration is 33 μM). When 1 ng of Cd2+ ions from this solution are released in 1 mL of receiving solution per hour, 400 ng of Cd2+ are actually released from the total QDs investigated because the volume of the dialysis solution is 400 mL. This corresponds to a concentration increment of 200 μg/L/h. This means that the amount of Cd2+ released in 10 nM of QDs is 61 ng/1000 g/h, which corresponds to 0.061 ppb/h. Since we removed the solution after 15 h for our experiment, this means that the concentration of Cd2+ is 0.91 ppb under our experimental conditions.
However, we know that Cd source, Te source, and thiol molecules form clusters upon aqueous synthesis of CdTe QDs [B]. Therefore, it is plausible that Cd2+ is released in the form of clusters from QDs. The very high concentration of QD solution (6.6 μM) upon dialysis, which is close to the concentration just after preparation [C], may further promote the formation of clusters. The size of the clusters can reach ~ 1 nm. Therefore, we suspect that the size of the pore for dialysis (500 MWCO) is too small to precisely evaluate the total amount of Cd2+ released. Another reported dialysis of TGA-capped CdTe uses dialysis tubing with 6000- or 8000 MWCO [D]. As described in the text, our selection for measurement was 3000 MWCO for the CdSe-related QDs, which is slightly smaller than the size of the QD itself. Our measurement of Cd2+ from red-emitting TGA-capped CdTe was 80 ppb after 15 h in pure water, which is similar to the value (30 ppb) discussed in the following section on HeLa cells.
Concentration of released Cd2+ in Su Y et al., Biomaterials, 31, 4829–4834 (2010)
Since the emission of the QD is in the red region, we assumed the diameter of CdTe to be 3.8 nm. In this case, 420 Cd atoms are contained in each QD if the number of Cd and Te atoms is the same. Since the HeLa cell volume is estimated to be 3000 μm3 [E], the concentration of free Cd2+ in cells containing 1 ng/105 cells corresponds to 33 ppb.
Because the intracellular Cd of CSS (CdTe/CdS/ZnS) is in the order of 1 ng/105 cells (Fig. 3b), the concentration of QD is roughly 70 nM. Intracellular Cd2+ for CSS is in the order of 0.1 ng/105 cells (Fig. 5b). Therefore, its concentration is 330 ppb. The duration of dispersion is 24 h. This therefore corresponds to ~ 30 ppb for a 10-nM dispersion of QDs over 15 h (our conditions).
References in appendices
[A] Murase N, Gaponik N, Weller H (2007) Effect of chemical composition on luminescence of thiol-stabilized CdTe nanocrystals. Nanoscale Res Lett 2:230–234
[B] Shavel A, Gaponik N, Eychmüller A (2006) Factors governing the quality of aqueous CdTe nanocrystals: calculations and experiment. J Phys Chem B 110, 19:280–19,284
[C] Li CL, Murase N (2005) Surfactant-dependent photoluminescence of CdTe nanocrystals in aqueous solution. Chem Lett 34:92–93
[D] Gao MY, Kirstein S, Möhwald H, Rogach AL, Kornowski A, Eychmüller A, Weller H (1998) Strongly photoluminescent CdTe nanocrystals by proper surface modification. J Phys Chem B 102:8360–8363
Rights and permissions
About this article
Cite this article
Murase, N., Horie, M., Sawai, T. et al. Silica layer-dependent leakage of cadmium from CdSe/ZnS quantum dots and comparison of cytotoxicity with polymer-coated analogues. J Nanopart Res 21, 10 (2019). https://doi.org/10.1007/s11051-018-4449-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-018-4449-2