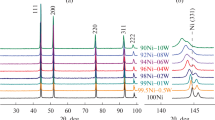
Abstract
Comprehensive understanding of the hydrogen gas interaction with metal nanoparticles is crucial for the development of multifunctional materials. The hydrogen absorption properties of well-dispersed Pd–Ir nanoalloys on a mesoporous carbon are reported here. The average size of nanoalloys depends on the composition and is comprised between 2.7 and 3.5 nm with decreasing Ir content. Structural analysis evidences a single phase FCC structure for all nanoparticles and a linear variation of the lattice parameter with composition confirming the formation of nanoalloys in this bulk-immiscible system. The hydrogen absorption properties can be tuned by the chemical composition: Pd-rich nanoparticles form hydride phases, whereas Ir-rich phases do not absorb hydrogen under ambient temperature and pressure conditions. The thermodynamic properties of hydride formation in Pd-rich phases are altered relative to the bulk counterparts. Moreover, the hydrogen absorption capacity in Pd-rich nanoalloys is larger as compared to bulk alloys. This might be explained by an important finite size effect that increases the hydrogen absorption capability of Pd–Ir alloys at nanoscale.






Similar content being viewed by others
References
Akamaru S, Hara M, Matsuyama M (2014) Alloying effects on the hydrogen-storage capability of Pd-TM-H (TM = Cu, Au, Pt, Ir) systems. J Alloys Compd 614:238–243. doi:10.1016/j.jallcom.2014.06.118
Bérubé V, Radtke G, Dresselhaus M, Chen G (2007) Size effects on the hydrogen storage properties of nanostructured metal hydrides: a review. Int J Energy Res 31:637–663
Bogerd R, Adelhelm P, Meeldijk JH et al (2009) The structural characterization and H2 sorption properties of carbon-supported Mg(1-x)Nix nanocrystallites. Nanotechnology 20:204019
de Jongh PE, Adelhelm P (2010) Nanosizing and nanoconfinement: new strategies towards meeting hydrogen storage goals. ChemSusChem 3:1332–1348
de Jongh PE, Allendorf M, Vajo JJ, Zlotea C (2013) Nanoconfined light metal hydrides for reversible hydrogen storage. MRS Bull 38:488–494. doi:10.1557/mrs.2013.108
Driessen AA, Sanger PP, Hemmes HH, Griessen R (1990) Metal hydride formation at pressures up to 1Mbar. J Phys-Condens Matter 2:9797–9814. doi:10.1088/0953-8984/2/49/007
Ghimbeu CM, Zlotea C, Gadiou R et al (2011) Understanding the mechanism of hydrogen uptake at low pressure in carbon/palladium nanostructured composites. J Mater Chem 21:17765–17775. doi:10.1039/c1jm12939b
Griessen R, Strohfeldt N, Giessen H (2016) Thermodynamics of the hybrid interaction of hydrogen with palladium nanoparticles. Nat Mater 15:311–317
Kittel C (1996) Introduction to solid state physics. Wiley
Kobayashi H, Yamauchi M, Kitagawa H (2012) Finding hydrogen-storage capability in iridium induced by the nanosize effect. J Am Chem Soc 134:6893–6895
LaPrade M, Allard KD, Lynch JF, Flanagan TB (1974) Absorption of hydrogen by iridium/palladium substitutional alloys. J Chem Soc Faraday Trans 1(70):1615–1630. doi:10.1039/F19747001615
Lewis FA (1982) The palladium-hydrogen system. Platin Met Rev 26:121–128
Liu X, Han Y, Evans JW et al (2015) Growth morphology and properties of metals on graphene. Prog Surf Sci 90:397–443. doi:10.1016/j.progsurf.2015.07.001
Liu X, Wang CZ, Hupalo M et al (2012) Metals on graphene: correlation between adatom adsorption behavior and growth morphology. Phys Chem Chem Phys 14:9157–9166. doi:10.1039/C2CP40527J
Manadé M, Viñes F, Illas F (2015) Transition metal adatoms on graphene: a systematic density functional study. Carbon 95:525–534. doi:10.1016/j.carbon.2015.08.072
Massalki TB (1990) Binary alloy phase diagrams, Second Edition. Ohio
Morfin F, Nassreddine S, Rousset JL, Piccolo L (2012) Nanoalloying effect in the preferential oxidation of CO over Ir-Pd catalysts. ACS Catal 2:2161–2168. doi:10.1021/cs3003325
Noh H, Luo S, Wang D et al (1995) The effects of hydriding-dehydriding cycles on the plateau pressures and van’t Hoff plots for Pd-Ni alloys. J Alloys Compd 218:139–142. doi:10.1016/0925-8388(94)01404-3
Oates WA (1982) Thermodynamic properties of the Pd-H system. J Common Met 88:411–424
Oumellal Y, Ghimbeu CM, de Yuso AM, Zlotea C (2017) Hydrogen absorption properties of carbon supported Pd–Ni nanoalloys. Int J Hydrog Energy. doi:10.1016/j.ijhydene.2016.09.101
Oumellal Y, Joubert J-M, Ghimbeu CM et al (2016a) Synthesis and stability of Pd–Rh nanoalloys with fully tunable particle size and composition. Nano-Struct Nano-Objects 7:92–100. doi:10.1016/j.nanoso.2016.06.005
Oumellal Y, Provost K, Ghimbeu CM et al (2016b) Composition and size dependence of hydrogen interaction with carbon supported bulk-immiscible Pd–Rh nanoalloys. Nanotechnology 27:465401
Piccolo L (2012) Surface studies of catalysis by metals: nanosize and alloying effects. In: Nanoalloys: synthesis, structure and properties, Springer-Verlag. London,
Piccolo L, Nassreddine S, Aouine M et al (2012) Supported Ir–Pd nanoalloys: size–composition correlation and consequences on tetralin hydroconversion properties. J Catal 292:173–180. doi:10.1016/j.jcat.2012.05.010
Pundt A, Kirchheim R (2006) Hydrogen in metals: microstructural Aspects. Annu Rev Mater Res 36:555–608. doi:10.1146/annurev.matsci.36.090804.094451
Raub E (1959) Metals and alloys of the platinum group. J Common Met 1:3–18. doi:10.1016/0022-5088(59)90014-1
Roduner E (2006) Size matters: why nanomaterials are different. Chem Soc Rev 35:583–592. doi:10.1039/B502142C
Scheler T, Marqués M, Konôpková Z et al (2013) High-pressure synthesis and characterization of iridium trihydride. Phys Rev Lett 111:215503. doi:10.1103/PhysRevLett.111.215503
Vajo J, Pinkerton F, Stetson N (2009) Nanoscale phenomena in hydrogen storage. Nanotechnology 20:200201
Vons VA, Leegwater H, Legerstee WJ et al (2010) Hydrogen storage properties of spark generated palladium nanoparticles. Int J Hydrog Energy 35:5479–5489. doi:10.1016/j.ijhydene.2010.02.118
Weissmüller J, Lemier C (1999) Lattice constants of solid solution microstructures: the case of nanocrystalline Pd-H. Phys Rev Lett 82:213–216. doi:10.1103/PhysRevLett.82.213
Weissmuller J, Lemier C (2000) On the size dependence of the critical point of nanoscale interstitial solid solutions. Philos Mag Lett 80:411–418. doi:10.1080/095008300403558
Yamauchi M, Ikeda R, Kitagawa H, Takata M (2008) Nanosize effects on hydrogen storage in palladium. J Phys Chem C 112:3294–3299. doi:10.1021/jp710447j
Zlotea C, Cuevas F, Andrieux J et al (2013) Tunable synthesis of (Mg-Ni)-based hydrides nanoconfined in templated carbon studied by in situ synchrotron diffraction. Nano Energy 2:12–20. doi:10.1016/j.nanoen.2012.07.005
Zlotea C, Cuevas F, Paul-Boncour V et al (2010) Size-dependent hydrogen sorption in ultrasmall Pd clusters embedded in a mesoporous carbon template. J Am Chem Soc 132:7720–7729. doi:10.1021/ja101795g
Zlotea C, Ghimbeu CM, Oumellal Y et al (2015a) Hydrogen sorption properties of Pd-Co nanoalloys embedded into mesoporous carbons. Nanoscale 7:15469–15476. doi:10.1039/C5NR03143E
Zlotea C, Latroche M (2013) Role of nanoconfinement on hydrogen sorption properties of metal nanoparticles hybrids. Colloids Surf Physicochem Eng Asp 439:117–130. doi:10.1016/j.colsurfa.2012.11.043
Zlotea C, Morfin F, Nguyen TS et al (2014) Nanoalloying bulk-immiscible iridium and palladium inhibits hydride formation and promotes catalytic performances. Nanoscale 6:9955–9959. doi:10.1039/c4nr02836h
Zlotea C, Oumellal Y, Hwang S-J et al (2015b) Ultrasmall MgH2 nanoparticles embedded in an ordered microporous carbon exhibiting rapid hydrogen sorption kinetics. J Phys Chem C 119:18091–18098. doi:10.1021/acs.jpcc.5b05754
Zlotea C, Oumellal Y, Msakni M et al (2015c) First evidence of Rh nano-hydride formation at low pressure. Nano Lett 15:4752–4757. doi:10.1021/acs.nanolett.5b01766
Zlotea C, Oumellal Y, Provost K, Matei Ghimbeu C (2016) Experimental challenges in studying hydrogen absorption in ultra-small metal nanoparticles. Front Energy Res 4:24. doi:10.3389/fenrg.2016.00024
Acknowledgements
Julie Bourgon is acknowledged for TEM measurements.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by the French National Research Agency (ANR) under GENESIS contract no 13-BS08-0004.
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Malouche, A., Oumellal, Y., Ghimbeu, C.M. et al. Exploring the hydrogen absorption into Pd–Ir nanoalloys supported on carbon. J Nanopart Res 19, 270 (2017). https://doi.org/10.1007/s11051-017-3978-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-017-3978-4