Abstract
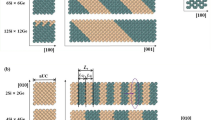
Engineering nanowires to develop new products and processes is highly topical due to their ability to provide highly enhanced physical, chemical, mechanical, thermal and electrical properties. In this work, using molecular dynamics simulations, we report fundamental information, about the structural and thermodynamic properties of individual anatase titania (TiO2) nanowires with cross-sectional diameters between 2 and 6 nm, and aspect ratio (length to diameter) of 6:1 at temperatures ranging from 300 to 3000 K. Estimates of the melting transition temperature of the nanowires are between 2000 and 2500 K. The melting transition temperature predicted from the radial distribution functions (RDFs) shows strong agreement with those predicted from the total energy profiles. Overall, the transition temperature is in reasonable agreement with melting points predicted from experiments and simulations reported in the literature for spherical nanoparticles of similar sizes. Hence, the melting transition temperature of TiO2 nanowires modelled here can be considered as shape independent. Furthermore, for the first time based on MD simulations, interaction forces between two nanowires are reported at ambient temperature (300 K) for different orientations: parallel, perpendicular and end-to-end. It is observed that end-to-end orientations manifested the strongest attraction forces, while the parallel and perpendicular orientations displayed weaker attractions. The results reported here could form a foundation in future multiscale modelling studies of the structured titania nanowire assemblies, depending on the inter-wire interaction forces.

ᅟ








Similar content being viewed by others
References
Abdullah H, Kuo D-H, Kuo Y-R, Yu F-A, Cheng K-B (2016) Facile synthesis and recyclability of thin nylon film-supported n-type ZnO/p-type Ag2O nano composite for visible light photocatalytic degradation of organic dye. J Phys Chem C 120(13):7144–7154
Acharya S, Efrima S (2005) Two-dimensional pressure-driven nanorod-to-nanowire reactions in Langmuir monolayers at room temperature. J Am Chem Soc 127(10):3486–3490
Banfield JF, Bischoff BL, Anderson MA (1993) TiO2 accessory minerals: coarsening, and transformation kinetics in pure and doped synthetic nanocrystalline materials. Chem Geol 110(1–3):211–231
Berendsen HJC, Postma JPM, van Gunsteren WF, DiNola A, Haak JR (1984) Molecular dynamics with coupling to an external bath. J Chem Phys 81(8):3684–3690
Binnig G, Quate CF, Gerber C (1986) Atomic force microscope. Phys Rev Lett 56(9):930–933
Boal AK, Ilhan F, DeRouchey JE, Thurn-Albrecht T, Russell TP, Rotello VM (2000) Self-assembly of nanoparticles into structured spherical and network aggregates. Nature 404(6779):746–748
Brostow W (1977) Radial distribution function peaks and coordination numbers in liquids and in amorphous solids. Chem Phys Lett 49(2):285–288
Chaudhari GN, Bambole DR, Bodade AB, Padole PR (2006) Characterization of nanosized TiO2 based H2S gas sensor. J Mater Sci 41(15):4860–4864
Chen X, Mao SS (2006) Synthesis of titanium dioxide (TiO2) nanomaterials. J Nanosci Nanotechnol 6(4):906–925
De Jong WH, Borm PJA (2008) Drug delivery and nanoparticles: applications and hazards. Int J Nanomedicine 3(2):133–149
Ding Y, Chen H, Musina Z, Jin Y, Zhang T, Witharana S, Yang W (2010) Relationship between the thermal conductivity and shear viscosity of nanofluids. Phys Scr T139(2010)
Dong KJ, Yang RY, Zou RP, Yu AB (2006) Role of interparticle forces in the formation of random loose packing. Phys Rev Lett 96(14):145505
Duncan R (2003) The dawning era of polymer therapeutics. Nat Rev Drug Discov 2(5):347–360
Ferrari M (2005) Cancer nanotechnology: opportunities and challenges. Nat Rev Cancer 5(3):161–171
Filyukov D, Brodskaya E, Piotrovskaya E, de Leeuw S (2007) Molecular-dynamics simulation of nanoclusters of crystal modifications of titanium dioxide. Russ J Gen Chem 77(1):10–16
Francioso L, Taurino AM, Forleo A, Siciliano P (2008) TiO2 nanowires array fabrication and gas sensing properties. Sensors Actuators B Chem 130(1):70–76
Garg P, Alvarado JL, Marsh C, Carlson TA, Kessler DA, Annamalai K (2009) An experimental study on the effect of ultrasonication on viscosity and heat transfer performance of multi-wall carbon nanotube-based aqueous nanofluids. Int J Heat Mass Transf 52(21–22):5090–5101
Gorkhover T, Schorb S, Coffee R, Adolph M, Foucar L, Rupp D, Aquila A, Bozek JD, Epp SW, Erk B, Gumprecht L, Holmegaard L, Hartmann A, Hartmann R, Hauser G, Holl P, Hömke A, Johnsson P, Kimmel N, Kühnel K-U, Messerschmidt M, Reich C, Rouzée A, Rudek B, Schmidt C, Schulz J, Soltau H, Stern S, Weidenspointner G, White B, Küpper J, Strüder L, Schlichting I, Ullrich J, Rolles D, Rudenko A, Möller T, Bostedt C (2016) Femtosecond and nanometre visualization of structural dynamics in superheated nanoparticles. Nat Photon 10(2):93–97
Gudiksen MS, Wang J, Lieber CM (2001) Synthetic control of the diameter and length of single crystal semiconductor nanowires. J Phys Chem B 105(19):4062–4064
Haverkamp RG (2010) A decade of nanoparticle research in Australia and New Zealand. Part Sci Technol: An Int J 28(1):1–40
Heinz H, Suter U (2004) Atomic charges for classical simulations of polar systems. J Phys Chem B 108(47):18341–18352
Hines A, Walls H, Jethani K (1985) Determination of the coordination number of liquid metals near the melting point. Metall Trans A 16(1):267–274
Hoang VV (2008) The glass transition and thermodynamics of liquid and amorphous TiO 2 nanoparticles. Nanotechnology 19(10):105706
Horn M, Schwerdtfeger C, Meagher E (1972) Refinement of the structure of anatase at several temperatures. Z Krist 136(3–4):273
Hwang YJ, Hahn C, Liu B, Yang P (2012) Photoelectrochemical properties of TiO2 nanowire arrays: a study of the dependence on length and atomic layer deposition coating. ACS Nano 6(6):5060–5069
Jagtap N, Bhagwat M, Awati P, Ramaswamy V (2005) Characterization of nanocrystalline anatase titania: an in situ HTXRD study. Thermochim Acta 427(1–2):37–41
Jono K, Ichikawa H, Miyamoto M, Fukumori Y (2000) A review of particulate design for pharmaceutical powders and their production by spouted bed coating. Powder Technol 113(3):269–277
Karmakar S, Kumar S, Rinaldi R, Maruccio G (2011) Nano-electronics and spintronics with nanoparticles. J Phys Conf Ser 292(1):012002
Koparde VN, Cummings PT (2005) Molecular dynamics simulation of titanium dioxide nanoparticle sintering. J Phys Chem B 109(51):24280–24287
Koparde VN, Cummings PT (2007) Molecular dynamics study of water adsorption on TiO2 nanoparticles. J Phys Chem C 111(19):6920–6926
Kulkarni A, Rao P, Natarajan S, Goldman A, Sabbisetti VS, Khater Y, Korimerla N, Chandrasekar V, Mashelkar RA, Sengupta S (2016) Reporter nanoparticle that monitors its anticancer efficacy in real time. Proc Natl Acad Sci 113(15):E2104–E2113
Larson I, Drummond CJ, Chan DYC, Grieser F (1993) Direct force measurements between titanium dioxide surfaces. J Am Chem Soc 115(25):11885–11890
Laube L, Salameh, S, Kappl M, Mädler L, Ciacchi LC (2015) Contact forces between TiO2 nanoparticles governed by an interplay of adsorbed water layers and roughness. Langmuir 31(41):11288–11295
Leach A (2001) Molecular modelling: principles and applications, 2nd edn. Prentice Hall, London
Lin H, Li L, Zhao M, Huang X, Chen X, Li G, Yu R (2012) Synthesis of high-quality Brookite TiO2 single-crystalline nanosheets with specific facets exposed: tuning catalysts from inert to highly reactive. J Am Chem Soc 134(20):8328–8331
Mahshid S, Askari M, Ghamsari MS (2007) Synthesis of TiO2 nanoparticles by hydrolysis and peptization of titanium isopropoxide solution. J Mater Process Technol 189(1–3):296–300
Materials Studio suite of crystallographic programs [Online]. Available from http://accelrys.com/products/collaborative-science/biovia-materials-studio/
Matsui M, Akaogi M (1991) Molecular dynamics simulation of the structural and physical properties of the four polymorphs of TiO2. Mol Simul 6(4–6):239–244
Min Y, Akbulut M, Kristiansen K, Golan Y, Israelachvili J (2008) The role of interparticle and external forces in nanoparticle assembly. Nat Mater 7(7):527–538
Mohd Azlishah O, Noor Faridah A, Badrul Hisham A, Jose R (2014) Electrical conductivity characteristic of TiO2 nanowires from hydrothermal method. J Phys Conf Ser 495(1):012027
Moshofsky B, Mokari T (2013) Length and diameter control of ultrathin nanowires of substoichiometric tungsten oxide with insights into the growth mechanism. Chem Mater 25(8):1384–1391
Naicker PK, Cummings PT, Zhang H, Banfield JF (2005) Characterization of titanium dioxide nanoparticles using molecular dynamics simulations. J Phys Chem B 109(32):15243–15249
Okeke G, Witharana S, Antony S, Ding Y (2011) Computational analysis of factors influencing thermal conductivity of nanofluids. J Nanopart Res 13(12):6365–6375
Okeke G, Hammond RB, Antony SJ (2013) Molecular dynamics simulation of anatase TiO2 nanoparticles. J Nanosci Nanotechnol 13(2):1047–1052
Okeke G, Hammond RB, Antony SJ (2016) Effects of heat treatment on the atomic structure and surface energy of rutile and anatase TiO2 nanoparticles under vacuum and water environments. Chem Eng Sci 146:144–158
Oliver PM, Watson GW, Toby Kelsey E, Parker SC (1997) Atomistic simulation of the surface structure of the TiO2 polymorphs rutileand anatase. J Mater Chem 7(3):563–568
Park S-J, Kang YC, Park JY, Evans EA, Ramsier RD, Chase GG (2010) Physical characteristics of Titania nanofibers synthesized by sol-gel and electrospinning techniques. J Eng Fibers Fabr 5(1):50–56
Pradhan N, Efrima S (2004) Supercrystals of uniform nanorods and nanowires, and the nanorod-to-nanowire oriented transition. J Phys Chem B 108(32):11964–11970
Pritesh H, Husnu Emrah U, Gehan AJA (2012) Nanowires for energy generation. Nanotechnology 23(19):194002
Ramirez-Garcia S, Chen L, Morris MA, Dawson KA (2011) A new methodology for studying nanoparticle interactions in biological systems: dispersing titania in biocompatible media using chemical stabilisers. Nano 3(11):4617–4624
Salameh S, Schneider J, Laube J, Alessandrini A, Facci P, Seo JW, Ciacchi LC, Mädler L (2012) Adhesion mechanisms of the contact interface of TiO2 nanoparticles in films and aggregates. Langmuir 28(31):11457–11464
Shirale DJ, Bangar MA, Chen W, Myung NV, Mulchandani A (2010) Effect of aspect ratio (length:diameter) on a single polypyrrole nanowire FET device. J Phys Chem C 114(31):13375–13380
Smith W, Forester TR (1996) DL_POLY_2.0: a general-purpose parallel molecular dynamics simulation package. J Mol Graph 14(3):136–141
Smith W, Forester TR, Todorov IT (2010) The DL_POLY_2 User Manual. STFC Daresbury Laboratory. Version 2.21
Tang H, Berger H, Schmid PE, Lévy F, Burri G (1993) Photoluminescence in TiO2 anatase single crystals. Solid State Commun 87(9):847–850
Xu ZP, Zeng QH, Lu GQ, Yu AB (2006) Inorganic nanoparticles as carriers for efficient cellular delivery. Chem Eng Sci 61(3):1027–1040
Zeng Q, Yu A, Lu G (2010) Evaluation of interaction forces between nanoparticles by molecular dynamics simulation. Ind Eng Chem Res 49(24):12793–12797
Zhang H, Chen B, Banfield JF, Waychunas GA (2008) Atomic structure of nanometer-sized amorphous TiO_{2}. Phys Rev B 78(21):214106
Zheng L, Xu M, Xu T (2000) TiO2−x thin films as oxygen sensor. Sensors Actuators B Chem 66(1–3):28–30
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Okeke, G., Antony, S.J., Hammond, R.B. et al. Structures and orientation-dependent interaction forces of titania nanowires using molecular dynamics simulations. J Nanopart Res 19, 237 (2017). https://doi.org/10.1007/s11051-017-3930-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-017-3930-7