Abstract
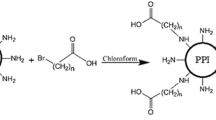
Aimed to prepare an enhanced gene delivery system with low cytotoxicity and high transfection efficiency, various cholesterol-conjugated derivates of low generation polyamidoamine (PAMAM) dendrimers were prepared. The conjugates were characterized by TNBS assay, FTIR, and 1H-NMR spectroscopy. Self-assembly of the dendrimer conjugates (G1-Chol, G2-Chol, and G3-Chol) was investigated by pyrene assay. Following formation of the complexes between enhanced green fluorescence protein plasmid and the dendrimer conjugates at various N (primary amine)/P (phosphate) mole ratios, plasmid condensation, biologic stability, cytotoxicity, and protein expression were investigated. The conjugates self-assembled into micellar dispersions with the critical micelle concentration values (<50 µg/ml) depending on the dendrimer generation and cholesterol/amine mole ratio. Cholesterol conjugation resulted in higher resistance of the condensed plasmid DNA in a competition assay with heparin sulfate. Also, the transfection efficiency was determined higher for the cholesterol conjugates than unmodified dendrimers in HepG2 cells, showing the highest for G2-Chol at 40 % degree of cholesterol modification (G2-Chol40 %) among various dendrimer generations. Interestingly, such conjugate showed a complete protection of plasmid against serum nucleases. Our results confirmed that the cholesterol conjugation to PAMAM dendrimers of low generations bearing little cytotoxicity improves their several physicochemical and biological characteristics required for an enhanced delivery of plasmid DNA into cells.









Similar content being viewed by others
References
Alshamsan A, Haddadi A, Incani V, Samuel J, Lavasanifar A, Uludag H (2009) Formulation and delivery of siRNA by oleic acid and stearic acid modified polyethylenimine. Mol Pharm 6:121–133
Bielinska A, Kukowska-Latallo JF, Johnson J, Tomalia DA, Baker JR (1996) Regulation of in vitro gene expression using antisense oligonucleotides or antisense expression plasmids transfected using starburst PAMAM dendrimers. Nucleic Acids Res 24:2176–2182
Bielinska AU, Chen C, Johnson J, Baker JR (1999) DNA complexing with polyamidoamine dendrimers implications for transfection. Bioconjug Chem 10:843–850
Biswas S, Torchilin VP (2013) Dendrimers for siRNA Delivery. Pharmaceuticals 6:161–183
Biswas S, Deshpande PP, Navarro G, Dodwadkar NS, Torchilin VP (2013) Lipid modified triblock PAMAM-based nanocarriers for siRNA drug co-delivery. Biomaterials 34:1289–1301
Charest MC, Rhainds D, Falstrault L, Matzouranis T, Brissett L (1999) Selective uptake of cholesteryl ester from low density lipoprotein is involved in HepG2 cell cholesterol homeostasis. Eur J Biochem 263:402–409
Chen W, Turro NJ, Tomalia DA (2000) Using ethidium bromide to probe the interactions between DNA and dendrimers. Langmuir 16:15–19
Choi JS, Ko KS, Park JS, Kim YH, Kim SW, Lee M (2006) Dexamethasone conjugated poly(amidoamine) dendrimer as a gene carrier for efficient nuclear translocation. Int J Pharm 320:171–178
Dehshahri A, Oskuee RK, Shier WT, Hatefi A, Ramezani M (2009) Gene transfer efficiency of high primary amine content, hydrophobic, alkyl-oligoamine derivatives of polyethylenimine. Biomater 30:4187–4194
Deniz A, Sade A, Severcan F, Keskin D, Tezcaner A, Banerjee S (2010) Celecoxib-loaded liposomes: effect of cholesterol on encapsulation and in vitro release characteristics. Biosci Rep 30:365–373
Dong X, Liu C (2010) Preparation and characterization of self-assembled nanoparticles of hyaluronic acid-deoxycholic acid conjugates. J Nanomater 12:1–9
Dung TH, Kim JS, Juliano RL, Yoo H (2008) Preparation and evaluation of cholesteryl PAMAM dendrimers as nano delivery agents for antisense oligonucleotides. Colloids Surf A 313–314:273–277
Escoffre J-M, Teissié J, Rols M-P (2010) Gene transfer: how can the biological barriers be overcome? J Membr Biol 236:61–74
Fant K, Esbjörner EK, Jenkins A, Grossel MC, Lincoln P, Nordén B (2010) Effects of PEGylation and acetylation of PAMAM dendrimers on DNA binding, cytotoxicity and in vitro transfection efficiency. Mol Pharm 7:1734–1746
Fitzsimmons REB, Uludağ H (2012) Specific effects of PEGylation on gene delivery efficacy of polyethylenimine: interplay between PEG substitution and N/P ratio. Acta Biomater 8:3941–3955
Freedman RB, Radda GK (1968) The reaction of 2,4,6-trinitrobenzenesulphonic acid with amino acids, peptides and proteins. Biochem J 108:383–391
Gao X, Huang L (1991) A novel cationic liposome reagent for efficient transfection of mammalian cells. Biochem Biophys Res Commun 179:280–285
Gao X, Kim K-S, Liu D (2007) Nonviral gene delivery: what we know and what is next. AAPS J 9:E92–E104
Geall AJ, Blagbrough IS (2000) Rapid and sensitive ethidium bromide fluorescence quenching assay of polyamine conjugate–DNA interactions for the analysis of lipoplex formation in gene therapy. J Pharm Biomed Anal 22:849–859
Gersdorff KV, Ogris M, Wagner E (2005) Cryoconserved shielded and EGF receptor targeted DNA polyplexes: cellular mechanisms. Eur J Pharm Biopharm 60:279–285
Guo S, Huang L (2011) Nanoparticles escaping RES and endosome: challenges for siRNA delivery for cancer therapy. J Nanomater 5:1–12
Gusachenko Simonova O, Kravchuk Y, Konevets D, Silnikov V, Vlassov VV, Zenkova MA (2009) Transfection efficiency of 25-kDa PEI-cholesterol conjugates with different levels of modification. J Biomater Sci Polym Ed 20:1091–1110
Haensler J, Szoka FC Jr (1993) Polyamidoamine cascade polymers mediate efficient transfection of cells in culture. Bioconjugate Chem 4:372–379
Haririan I et al (2010) Cellular delivery of nanostructured poly(amido amine) dendrimers and establishment of a simple methodology upon ninhydrin reaction. Iran J Pharm Sci 6:71–82
Hashemi M, Sahraie Fard H, Amel Farzad S, Parhiz H, Ramezani M (2013) Gene transfer enhancement by alkylcarboxylation of poly(propylenimine). Nanomed J 1:55–62
Hirsch-Lernera D, Zhanga M, Eliyahua H, Ferrarib ME, Wheelerb CJ, Barenholza Y (2005) Effect of ‘‘helper lipid’’ on lipoplex electrostatics. Biochim Biophys Acta 1714(2):71–84
Hu Y et al (2003) Preparation and drug release behaviors of nimodipine-loaded poly(caprolactone)–poly(ethylene oxide)–polylactide amphiphilic copolymer nanoparticles. Biomater 24:2395–2404
Jafari M, Xu W, Pan R, Sweeting CM, Karunaratne DN, Chen P (2014) Serum stability and physicochemical characterization of a novel amphipathic peptide C6M1 for SiRNA delivery. PLoS One 9:e97797
Javitt NB (1990) Hep G2 cells as a resource for metabolic studies: lipoprotein, cholesterol, and bile acids. Faseb J 4:161–168
Jevprasesphant R, Penny J, Jalal R, Attwood D, McKeown NB, D’Emanuele A (2003) The influence of surface modification on the cytotoxicity of PAMAM dendrimers. Int J Pharm 252:263–266
Ju J, Huan M-L, Wan N, Qiu H, Zhou S-Y, Zhang B-L (2015) Novel cholesterol-based cationic lipids as transfecting agents of DNA for efficient gene delivery. Int J Mol Sci 16:5666–5681
Junquera E, Aicart E (2014) Cationic lipids as transfecting agents of DNA in gene therapy. Curr Top Med Chem 14:649–663
Kalyanasundaram K, Thomas J (1977) Environmental effects on vibronic band intensities in pyrene monomer fluorescence and their application in studies of micellar systems. J Am Chem Soc 99:2039–2044
Kamimura K, Suda T, Zhang G, Liu D (2011) Advances in gene delivery systems. Pharm Med 25:293–306
Kim TH, Yu GS, Choi H, Shim YJ, Lee M, Choi JS (2011) Preparation of dexamethasone-based cationic liposome and its application to gene delivery in vitro. J Nanosci Nanotech 11:1799–1802
Kleemann E et al (2005) Nano-carriers for DNA delivery to the lung based upon a TAT-derived peptide covalently coupled to PEG–PEI. J Control Release 109:299–316
Kukowska-Latallo JF, Bielinska AU, Johnson J, Spindler R, Tomalia DA, Baker JR (1996) Efficient transfer of genetic material into mammalian cells using Starburst polyamidoamine dendrimers. PNAS 93:4897–4902
Labieniec M, Watala C (2009) PAMAM dendrimers—diverse biomedical applications. Facts and unresolved questions. Centeur Biol 4:434–451
Lee ER et al (1996) Detailed analysis of structures and formulations of cationic lipids for efficient gene transfer to the lung. Hum Gene Ther 7:1701–1717
Lee M, Rentz J, Han SO, Bull DA, Kim SW (2003) Water-soluble lipopolymer as an efficient carrier for gene delivery to myocardium. Gene Ther 10:585–593
Lepecq JB, Paoletti C (1967) A fluorescent complex between ethidium bromide and nucleic acids: physical—chemical characterization. J Mol Biol 27:87–106
Liu C-G, Desai KGH, Chen X-G, Park H-J (2005) Linolenic acid-modified chitosan for formation of self-assembled nanoparticles. J Agric Food Chem 53:437–441
Liu C et al (2009) Novel biodegradable lipid nano complex for siRNA delivery significantly improving the chemosensitivity of human colon cancer stem cells to paclitaxel. J Control Release 140:277–283
Liu Z, Zhang Z, Zhou C, Jiao Y (2010a) Hydrophobic modifications of cationic polymers for gene delivery. Prog Polym Sci 35:1144–1162
Liu XQ, Du JZ, Zhang CP, Zhao F, Yang XZ, Wang J (2010b) Brush-shaped polycation with poly(ethylenimine)-b-poly(ethylene glycol) side chains as highly efficient gene delivery vector. Int J Pharm Sci 392:118–126
Mahato RI, Kim SW (2005) Water soluble lipopolymers for gene delivery. Polym Gene Deliv, CRC Press, Boca Raton
Mahato RI, Rolland A, Tomlinson E (1997) Cationic lipid-based gene delivery systems: pharmaceutical perspectives. Pharm Res 14:853–859
Malik N et al (2000) Dendrimers: relationship between structure and biocompatibility in vitro, and preliminary studies on the biodistribution of 125I-labelled polyamidoamine dendrimers in vivo. J Control Release 65:133–148
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63
Mukherjee SP, Byrne HJ (2013) Polyamidoamine dendrimer nanoparticle cytotoxicity, oxidative stress, caspase activation and inflammatory response: experimental observation and numerical simulation. Nanomed 9:202–211
Najafi H, Abolmaali S, Owrangi B, Ghasemi Y, Tamaddon A (2015) Serum resistant and enhanced transfection of plasmid DNA by PEG-stabilized polyplex nanoparticles of l-histidine substituted polyethyleneimine. Macromol Res 23:618–627
Nie Y, Ji L, Ding H, Xie L, Li L, He B, Wu Y, Gu Z (2012) Cholesterol derivatives based charged liposomes for doxorubicin delivery: preparation, in vitro and in vivo characterization. Theranostics 2:1092–1103
Park MR, Han KO, Han IK, Cho MHC, Nah WJ, Choi YJ, Cho CS (2009) Degradable polyethylenimine-alt-poly(ethylene glycol) copolymers as novel gene carriers. J Control Release 105:367–380
Perez AP, Romero EL, Morilla MJ (2009) Ethylendiamine core PAMAM dendrimers/siRNA complexes as in vitro silencing agents. Int J Pharm 380:189–200
Pozzi D, Marchini C, Cardarelli F, Amenitsch H, Garulli C, Bifone A, Caracciolo G (2012) Transfection efficiency boost of cholesterol-containing lipoplexes. Biochim Biophys Acta 1818:2335–2343
Raffy S, Teissie J (1999) Control of lipid membrane stability by cholesterol content. Biophys J 76:2072–2080
Remant Bahadur KC, Landry B, Aliabadi HM, Lavasanifar A, Uludağ H (2011) Lipid substitution on low molecular weight (0.6–2.0 kDa) polyethylenimine leads to a higher zeta potential of plasmid DNA and enhances transgene expression. Acta Biomater 7:2209–2217
Rezvani Amin Z, Rahimizadeh M, Eshghi H, Dehshahri A, Ramezani M (2013) The effect of cationic charge density change on transfection efficiency of polyethylenimine. Iran J Basic Med Sci 16:150–156
Sadekar S, Ghandehari H (2012) Transepithelial transport and toxicity of PAMAM dendrimers: implications for oral drug delivery. Adv Drug Deliv Rev 64:571–588
Santos JL, Pandita D, Rodrigues J, Pêgo AP, Granja PL, Balian G, Tomás H (2010) Receptor-mediated gene delivery using PAMAM dendrimers conjugated with peptides recognized by mesenchymal stem cells. Mol Pharm 7:763–774
Sara Movassaghian HRM, Shirazi Farshad H, Torchilin Vladimir P (2011) Dendrosome-dendriplex inside liposomes: as a gene delivery system. J Drug Tar 19:925–932
Sarkar K, Kundu PP (2012) Preparation of low molecular weight N-maleated chitosan-graft-PAMAM copolymer for enhanced DNA complexation. Int J Biol Macromol 51:859–867
Shah DS, Sakthivel T, Toth I, Florence AT, Wilderspin AF (2000) DNA transfection and transfected cell viability using amphipathic asymmetric dendrimers. Int J Pharm 208:41–48
Shigematsu M (1996) Concentration dependence of hydrophobicity of monosaccharides estimated by fluorescence of pyrene. Carbohydr Res 292:165–172
Shim MS, Kwon YJ (2010) Efficient and targeted delivery of siRNA in vivo. FEBS J 277:4814–4827
Snyder SL, Sobocinski PZ (1975) An improved 2, 4, 6-trinitrobenzenesulfonic acid method for the determination of amines. Anal Biochem 64:284–288
Han S-O, Mahato RI, Kim SW (2001) Water-soluble lipopolymer for gene delivery. Bioconjugate Chem 12:337–345
Stephen MS, Avery LM, Huan H, Kerstin KL, Gregory GM, Danilo LH, Ross P, Barbara PA, Ann BK, Friedhelm S (2012) Intracellular cholesterol-binding proteins enhance HDL-mediated cholesterol uptake in cultured primary mouse hepatocytes. Am J Physiol Gastrointest Liver Physiol 302:G824–G839
Sternberg B, Hong K, Zheng W, Papahadjopoulos D (1998) Ultrastructural characterization of cationic liposome–DNA complexes showing enhanced stability in serum and high transfection activity in vivo. Biochim Biophys Acta 1375(1–2):23–35
Swami A, Aggarwal A, Pathak A, Patnaik S, Kumar P, Singh Y, Gupta KC (2007) Imidazolyl-PEI modified nanoparticles for enhanced gene delivery. Int J Pharm 335:180–192
Tan Y-L, Liu C-G (2009) Self-aggregated nanoparticles from linoleic acid modified carboxymethyl chitosan: synthesis, characterization and application in vitro. Colloids Surf B 69:178–182
Takahashi T, Kono K, Itoh T, Emi N, Takagishi T (2003) Synthesis of novel cationic lipids having polyamidoamine dendrons and their transfection activity. Bioconje Chem 14:764–773
Tamaddon A, Hosseini-Shirazi F, Moghimi H (2007) Preparation of oligodeoxynucleotide encapsulated cationic liposomes and release study with models of cellular membranes. DARU J Pharm Sci 15:61–70
Tang MX, Redemann CT, Szoka FC (1996) In vitro gene delivery by degraded polyamidoamine dendrimers. Bioconjugate Chem 7:703–714
Tavakoli S, Tamaddon AM, Golkar N, Samani SM (2014) Microencapsulation of (deoxythymidine) 20-DOTAP complexes in stealth liposomes optimized by Taguchi design. J Liposome Res 25:67–77
Templeton N, Lasic D, Frederik P, Strey H, Roberts D, Pavlakis G (1997) Improved DNA: liposome complexes for increased systemic delivery and gene expression. Nat Biotechnol 15:647–652
Vigneron J-P et al (1996) Guanidinium-cholesterol cationic lipids: efficient vectors for the transfection of eukaryotic cells. PNAS 93:9682–9686
Wang DA, Narang AS, Kotb M, Gaber AO, Miller DD, Kim SW, Mahato RI (2002) Novel branched poly(ethylenimine)-cholesterol water-soluble lipopolymers for gene delivery. Biomacromol 3:1197–1207
Wang J, Lu Z, Wientjes MG, Au JLS (2010) Delivery of siRNA Therapeutics: barriers and Carriers. AAPS J 12:492–503
Yang Z, Sahay G, Sriadibhatla S, Kabanov AV (2008) Amphiphilic block copolymers enhance cellular uptake and nuclear entry of polyplex-delivered DNA. Bioconje Chem 19:1987–1994
Yang Y-L, Chang W-T, Shih Y-W (2011) Gene therapy using RNAi. INTECH open Access Publisher
Yoo H, Juliano R (2000) Enhanced delivery of antisense oligonucleotides with fluorophore-conjugated PAMAM dendrimers. Nucleic Acids Res 28:4225–4231
Yoo H, Sazani P, Juliano RL (1999) PAMAM dendrimers as delivery agents for antisense oligonucleotides. Pharm Res 16:1799–1804
Zhou J, Wu J, Hafdi N, Behr JP, Erbacher P, Peng L (2006) PAMAM dendrimers for efficient siRNA delivery and potent gene silencing. Chem Commun 22:2362–2364
Zhu L, Mahato RI (2010) Lipid and polymeric carrier-mediated nucleic acid delivery. Exp Opin Drug Deliv 7:1209–1226
Zuhorn I, Visser W, Bakowsky U, Engberts J, Hoekstra D (2002) Interference of serum with lipoplex–cell interaction: modulation of intracellular processing. Biochim Biophys Acta 1560:25–36
Acknowledgments
This work was supported financially by the grant from Shiraz University of Medical Sciences as a part of Ms. Nasim Golkar Ph.D. thesis. The facility support of “Center for Nanotechnology in Drug Delivery” is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Golkar, N., Samani, S.M. & Tamaddon, A.M. Cholesterol-conjugated supramolecular assemblies of low generations polyamidoamine dendrimers for enhanced EGFP plasmid DNA transfection. J Nanopart Res 18, 107 (2016). https://doi.org/10.1007/s11051-016-3413-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-016-3413-2